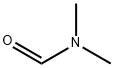
N,N-다이메틸폼아마이드
|
|
N,N-다이메틸폼아마이드 속성
- 녹는점
- -61 °C (lit.)
- 끓는 점
- 153 °C (lit.)
- 알파
- 0.94 º
- 밀도
- 0.944 g/mL (lit.)
- 증기 밀도
- 2.5 (vs air)
- 증기압
- 2.7 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.430(lit.)
- 인화점
- 136 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 물: 섞일 수 있음
- 산도 계수 (pKa)
- -0.44±0.70(Predicted)
- 물리적 상태
- 액체
- 색상
- APHA: ≤15
- 냄새
- 100ppm에서 희미한 암모니아 냄새가 감지됨
- 상대극성
- 0.386
- 수소이온지수(pH)
- 7 (200g/l, H2O, 20℃)
- 폭발한계
- 2.2-16%(V)
- Odor Threshold
- 1.8ppm
- 수용성
- 녹는
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 270 nm Amax: 1.00
λ: 275 nm Amax: 0.30
λ: 295 nm Amax: 0.10
λ: 310 nm Amax: 0.05
λ: 340-400 nm Amax: 0.01
- Merck
- 14,3243
- BRN
- 605365
- 노출 한도
- NIOSH REL: TWA 10 ppm (30 mg/m3), IDLH 500 ppm; OSHA PEL: TWA 10 ppm; ACGIH TLV: TWA 10 ppm (adopted).
- Dielectric constant
- 36.710000000000001
- InChIKey
- ZMXDDKWLCZADIW-UHFFFAOYSA-N
- LogP
- -1.010
- CAS 데이터베이스
- 68-12-2(CAS DataBase Reference)
- IARC
- 2A (Vol. 47, 71, 115) 2018
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 61-20/21-36 | ||
| 안전지침서 | 53-45 | ||
| 유엔번호(UN No.) | UN 2265 3/PG 3 | ||
| OEB | A | ||
| OEL | TWA: 10 ppm (30 mg/m3) [skin] | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | LQ2100000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 445 °C | ||
| 위험 참고 사항 | Toxic | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | III | ||
| HS 번호 | 29241990 | ||
| 유해 물질 데이터 | 68-12-2(Hazardous Substances Data) | ||
| 독성 | LD50 in mice, rats (ml/kg): 6.8, 7.6 orally; 6.2, 4.7 i.p. (Bartsch) | ||
| IDLA | 500 ppm | ||
| 기존화학 물질 | KE-11411 | ||
| 유해화학물질 필터링 | 2014-1-694 | ||
| 중점관리물질 필터링 | 별표1-10 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: N,N-디메틸포름아미드 및 이를 0.3% 이상 함유한 혼합물 |
N,N-다이메틸폼아마이드 C화학적 특성, 용도, 생산
용도
DMF의 주요 용도는 증발 속도가 낮은 용제입니다 .DMF는 아크릴 섬유 및 플라스틱 생산에 사용됩니다. 또한 의약품의 펩타이드 커플 링, 살충제의 개발 및 생산, 접착제, 합성기, 섬유, 필름 및 표면 코팅의 제조에 용매로 사용됩니다.용도
디메틸포름아미드는 특히 합성 피혁 제품의 생산을 위한 폴리우레탄 공정 영역에서 현저하게 많이 사용되고 있습니다. 작물 보호와 제약 산업에서는 활성 원료의 제조 시 사용됩니다. 또한 석유화학 산업에서는 벤젠이나 부타디엔 같은 특정한 제품의 추출을 위한 용도로 사용됩니다. 그 외에도 전기 산업에서는 회로 기판의 생산에 필요한 시재료의 제조 시 용매로서 사용됩니다.용도
N, N- 디메틸 포름 아미드 (N, N-Dimethyl Formamide, DMF)는 주로 의약품의 제조 또는 첨단 산업 분야의 용제로서 사용됩니다. DMF는 주로 공업용 용매로 사용됩니다. DMF 용액은 폴리머 섬유, 필름 및 표면 코팅을 처리하는 데 사용됩니다. 아크릴 섬유를 쉽게 방사 할 수 있도록; 와이어 에나멜을 생산하고, 제약 업계의 결정화 매체로 사용됩니다.용도
유기합성에서는 극성화합물의 용매로서 널리 쓰이는 외에 방향족화합물의 핵포르밀화, 기타 활성수소의 포르밀치환의 시약으로서 사용된다.화학적 성질
N,N-Dimethylformamide is a colorless or slightly yellow liquid with a boiling point of 153°C and a vapor pressure of 380 Pa at 20°C. It is freely soluble in water and soluble in alcohols, acetone and benzene. N,N-Dimethylformamide is used as solvent, catalyst and gas absorbent. React violently with concentrated sulfuric acid, fuming nitric acid and can even explode. Pure Dimethylformamide is odorless, but industrial grade or modified Dimethylformamide has a fishy smell because it contains impurities of Dimethylamine. Dimethylformamide is unstable (especially at high temperatures) in the presence of a strong base such as sodium hydroxide or a strong acid such as hydrochloric acid or sulfuric acid, and is hydrolyzed to formic acid and dimethylamine.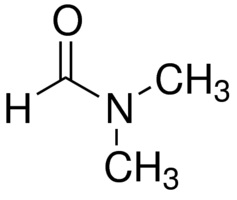
N,N-Dimethylformamide structure
물리적 성질
Clear, colorless to light yellow, hygroscopic, mobile liquid with a faint, characteristic, ammonialike odor. An experimentally determined odor threshold concentration of 100 ppmv was reported by Leonardos et al. (1969).용도
N,N-Dimethylformamide (DMF) is a clear liquid that has been widely used in industries as a solvent, an additive, or an intermediate because of its extensive miscibility with water and most common organic solvents. Dimethylformamide solutions are used toprocess polymer fibers, films, and surface coatings; to permit easy spinning of acrylic fibers; to produce wire enamels, and as a crystallization medium in the pharmaceutical industry.DMF can also be used for formylation with alkyllithium or Grignard reagents. It is used as a reagent in Bouveault aldehyde synthesis and also in Vilsmeier-Haack reaction. It acts as a catalyst in the synthesis of acyl chlorides. It is used for separating and refining crude from olefin gas. DMF along with methylene chloride acts as a remover of varnish or lacquers. It is also used in the manufacture of adhesives, fibers and films.
주요 응용
The principal applications of N,N-dimethylformamide are as a solvent and as an extractant, particularly for salts and compounds with high molecular mass. This role is consistent with its interesting combination of physical and chemical properties: low molecular mass, high dielectric constant, electron-donor characteristics, and ability to form complexes. The use of DMF as a component in synthesis is of relatively minor significance, at least commercially. N,N-Dimethylformamide (anhydrous) has been used as solvent for the synthesis of cytotoxic luteinizing hormone-releasing hormone (LH-RH) conjugate AN-152 (a chemotherapeutic drug) and fluorophore C625 [4-(N,N-diphenylamino)-4′-(6-O-hemiglutarate)hexylsulfinyl stilbene]. It may be employed as solvent medium for the various organic reduction reactions.생산 방법
Industrial production of N,N-Dimethylformamide (DMF) is via three separate processes (Eberling 1980). Dimethylamine in methanol is reacted with carbon monoxide in the presence of sodium methoxide or metal carbonyls at 110-150°C and high pressure. Alternately, methyl formate is produced from carbon monoxide and methanol under high pressure at 60-100°C in the presence of sodium methoxide. The resulting methyl formate is distilled and then reacted with dimethylamine at 80-100°C and low pressure. The third process involves reaction of carbon dioxide, hydrogen and dimethylamine in the presence of halogen-containing transition metal compounds to yield DMF.정의
ChEBI: N,N-dimethylformamide is a member of the class of formamides that is formamide in which the amino hydrogens are replaced by methyl groups. It has a role as a polar aprotic solvent, a hepatotoxic agent and a geroprotector. It is a volatile organic compound and a member of formamides. It is functionally related to a formamide.제조 방법
Two processes are used commercially to produce dimethylformamide. In the direct or one-step process, dimethylamine and carbon monoxide react at 100°C and 200 psia in the presence of a sodium methoxide catalyst to make dimethylformamide. The homogenous catalyst is separated from the crude DMF, which is then refined to the final product. In the indirect process, methyl formate is isolated, and then reacted with dimethylamine to form DMF. To obtain methyl formate, two methods may be used - dehydrogenation of methanol and esterification of formic acid.The two-step process for the synthesis of N,N-dimethylformamide differs from direct synthesis because methyl formate is prepared separately and introduced in the form of ca. 96% pure (commercialgrade) material. Equimolar amounts of methyl formate and N,N-dimethylamine are subjected to a continuous reaction at 60-100°C and 0.1 – 0.3 MPa. The resulting product is a mixture of N,N-dimethylformamide and methanol. The purification process involves distillation and is analogous to that described for direct synthesis. However, no separation of salts is required because no catalysts are involved in the process. According to the corrosive properties of both starting materials and products, stainless steel has to be used as material of construction for production facilities.
일반 설명
A water-white liquid with a faint fishy odor. Flash point 136°F. Slightly less dense than water. Vapors heavier than air. Toxic by inhalation or skin absorption. May irritate eyes.공기와 물의 반응
Flammable. Water soluble.반응 프로필
N,N-Dimethylformamide may react violently with a broad range of chemicals, e.g.: alkaline metals (sodium, potassium), azides, hydrides (sodium borohydride, lithium aluminum hydride), bromine, chlorine, carbon tetrachloride, hexachlorocyclohexane, phosphorus pentaoxide, triethylaluminum, magnesium nitrate, organic nitrates. Forms explosive mixtures with lithium azide [Bretherick, 5th ed., 1995, p. 453]. Oxidation by chromium trioxide or potassium permanganate may lead to explosion [Pal B. C. et al., Chem. Eng. News, 1981, 59, p. 47].건강위험
The acute toxicity of DMF is low by inhalation, ingestion, and skin contact. Contact with liquid DMF may cause eye and skin irritation. DMF is an excellent solvent for many toxic materials that are not ordinarily absorbed and can increase the hazard of these substances by skin contact. Exposure to high concentrations of DMF may lead to liver damage and other systemic effects. Dimethylformamide is listed by IARC in Group 2B ("possible human carcinogen"). It is not classified as a "select carcinogen" according to the criteria of the OSHA Laboratory Standard. No significant reproductive effects have been observed in animal tests. Repeated exposure to DMF may result in damage to the liver, kidneys, and cardiovascular system인화성 및 폭발성
DMF is a combustible liquid (NFPA rating = 2). Vapors are heavier than air and may travel to source of ignition and flash back. DMF vapor forms explosive mixtures with air at concentrations of 2.2 to 15.2% (by volume). Carbon dioxide or dry chemical extinguishers should be used to fight DMF fires.공업 용도
World production capacity of DMF is about 225 x 103 tons per year. The main application of DMF is as solvent in industrial processes, especially for polar polymers such as Polyvinylchloride, polyacrylonitrile and polyurethanes. DMF solutions of high molecular weight polymers are processed to fibers, films, surface coatings and synthetic leathers. Since salts can be dissolved and dissociated in DMF, the solutions are used in electrolytic capacitors and certain electrolytic processes (Eberling 1980).색상 색인 번호
This is an organic solvent for vinyl resins and acetylene, butadiene, and acid gases. It caused contact dermatitis in a technician at an epoxy resin factory and can provoke alcohol-induced flushing in exposed subjects.Carcinogenicity
DMF is not carcinogenic to animals except under very high inhalation exposure conditions. No increase in tumors was seen in rats that inhaled 25, 100, or 400 ppm for 6 h/day, 5 days/week for 2 years. Similarly, no tumors were produced in mice under the same conditions for 18 months. In that chronic experiment, rats and mice were exposed by inhalation (6 h/day, 5 days/week) to 0, 25, 100, or 400 ppm DMF for 18 months (mice) or 2 years (rats). Body weights of rats exposed to 100 (males only) and 400 ppm were reduced and, conversely, body weights were increased in 400 ppm mice. Serum sorbitol dehydrogenase activity was increased in rats exposed to 100 or 400 ppm. DMF-related morphological changes in rats were observed only in the liver and consisted of increased relative liver weights, centrilobular hepatocellular hypertrophy, lipofuscin/hemosiderin accumulation in Kupffer cells, and centrilobular single cell necrosis (400 ppm only). The same liver effects were seen in all groups of mice, although the response at 25 ppm was judged as minimal.환경귀착
Biological. Incubation of [14C]N,N-dimethylformamide (0.1–100 μg/L) in natural seawater resulted in the compound mineralizing to carbon dioxide. The rate of carbon dioxide formation was inversely proportional to the initial concentration (Ursin, 1985).Chemical. Reacts with acids or bases forming formic acid and dimethylamine (BASF, 1999)
신진 대사 경로
Three urinary metabolites are identified in humans and rodents, and the metabolites quantified are N- (hydroxymethyl)-N-methylformamide (HMMF), resulting in N-methylformamide (NMF) and N-acetyl-S-(N- methylcarbamoyl)cysteine (AMCC). Ten volunteers who absorb between 28 and 60 mmol/kg DMF during an 8 h exposure to DMF in air at 6 mg=m3 excrete in the urine within 72 h between 16.1 and 48.7% of the dose as HMMF, between 8.3 and 23.9% as formamide, and between 9.7 and 22.8% as AMCC. AMCC together with HMMF is also detected in the urine of workers after occupational exposure to DMF. There is a quantitative difference between the metabolic pathway of DMF to AMCC in humans and rodents.저장
DMF should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.비 호환성
Though stable at normal temperatures and storage conditions, DMF may react violently with halogens, acyl halides, strong oxidizers, and polyhalogenated compounds in the presence of iron. Decomposition products include toxic gases and vapors such as dimethylamine and carbon monoxide. DMF will attack some forms of plastics, rubber, and coatings.폐기물 처리
Excess DMF and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.N,N-다이메틸폼아마이드 준비 용품 및 원자재
원자재
준비 용품
EMITEFUR
Indole-5-carboxaldehyde
할로퓨진원
에틸2,4-디메틸퀴놀린-3-카르복실레이트
2,2'-비티오펜-5-카르복스알데하이드
4-BENZYLOXY-2-NITROTOLUENE
3-Chloro-2-hydroxy-5-nitropyridine
Pyridine-2-carbonyl chloride hydrochloride
1-(3-Pyridyl)-3-(dimethylamino)-2-propen-1-one
Bifendate
Olprinone
메틸프레드니솔론아세포네이트
4-메틸-2-(1H-PYRROL-2-YL)퀴놀린
1-[(4-메틸페닐)설포닐]-1H-인돌-3-카발데하이드
1-메틸-1H-피라졸로[3,4-B]피리딘-3-일라민
4-Acetamido-3-nitrobenzoic acid
9-DIETHYLAMINO-2-HYDROXY-5H-BENZ(A)-
2-Cyano-5-methylpyridine
Etomidate
a new kind of liquid crystal copolymer
ETHYL 2-(CHLOROMETHYL)-4-PHENYLQUINOLINE-3-CARBOXYLATE
3,5-Difluorophenylacetic acid
ETHYL 2-(CHLOROMETHYL)-4-PHENYLQUINOLINE-3-CARBOXYLATE HYDROCHLORIDE
2-(Aminomethyl)-1-ethylpyrrolidine
1-[2,6-DICHLORO-4-(TRIFLUOROMETHYL)PHENYL]-2,5-DIMETHYL-1H-PYRROLE-3-CARBALDEHYDE
2,3-(METHYLENEDIOXY)BENZALDEHYDE
4-AMINO-6-CHLORO-PYRIMIDINE-5-CARBALDEHYDE
N-METHYL-2-PIPERAZIN-1-YLACETAMIDE
Octenidine
4-(1H-IMIDAZOL-1-YL)벤조산
1-(1-메틸-4-피페리디닐)피페라진
2-CHLORO-4-(N,N-DIMETHYLAMINO)PYRIMIDINE
1-(2,4-DIMETHYLQUINOLIN-3-YL)ETHANONE HYDROCHLORIDE
(2-METHYL-4-PHENYLQUINOLIN-3-YL)ACETIC ACID HYDROCHLORIDE
Labetalol
Thiophene-3,4-dicarboxylic acid
RARECHEM알BI1318
세프피미졸
4,6-DIMETHOXYPYRIMIDINE-2-CARBONYL CHLORIDE
4,6-Dichloro-5-pyrimidinecarbaldehyde
N,N-다이메틸폼아마이드 공급 업체
글로벌( 1209)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 |
info@tnjchem.com | China | 2986 | 55 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17365 | 58 |
| Shandong Yanshuo Chemical Co., Ltd. | +86-18678179670 +86-18615116763 |
sales@yanshuochem.com | China | 101 | 58 |
| Hebei Miaoyin Technology Co.,Ltd | +86-17367732028 +86-17367732028 |
kathy@hbyinsheng.com | China | 3512 | 58 |
| Qingdao Minzhi Yijie new material Co., LTD | +86-13589435123 +86-13589435123 |
qdmzyj@126.com | China | 240 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 985 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8816 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 80 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5893 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-18033737140 +86-17354101231 |
sales1@hbganmiao.com | China | 227 | 58 |