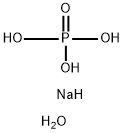
인산나트륨,이염기성,칠수화물
|
|
인산나트륨,이염기성,칠수화물 속성
- 녹는점
- 48 °C
- 밀도
- 1.68 g/mL at 25 °C(lit.)
- 증기 밀도
- 4.9 (vs air)
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 154g/L
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- Specific Gravity
- 1.7
- pH 범위
- 8.7 - 9.3
- 수소이온지수(pH)
- 8.7-9.3 (25℃, 5% in solution)
- 수용성
- 물에 용해되고 에탄올에 용해되지 않습니다.
- Merck
- 14,8659
- LogP
- -2.148 (est)
- CAS 데이터베이스
- 7782-85-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-24/25 | ||
| 유엔번호(UN No.) | UN 3077 9 / PGIII | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | WC4600000 | ||
| TSCA | Yes | ||
| HS 번호 | 28352990 | ||
| 독성 | LD50 orally in Rabbit: 12930 mg/kg |
인산나트륨,이염기성,칠수화물 C화학적 특성, 용도, 생산
용도
Buffering agent.생산 방법
Either bone phosphate (bone ash), obtained by heating bones to whiteness, or the mineral phosphorite is used as a source of tribasic calcium phosphate, which is the starting material in the industrial production of dibasic sodium phosphate.Tribasic calcium phosphate is finely ground and digested with sulfuric acid. This mixture is then leached with hot water and neutralized with sodium carbonate, and dibasic sodium phosphate is crystallized from the filtrate.
Pharmaceutical Applications
Dibasic sodium phosphate is used in a wide variety of pharmaceutical formulations as a buffering agent and as a sequestering agent. Therapeutically, dibasic sodium phosphate is used as a mild laxative and in the treatment of hypophosphatemia.Dibasic sodium phosphate is also used in food products; for example as an emulsifier in processed cheese.
Safety
Dibasic sodium phosphate is widely used as an excipient in parenteral, oral, and topical pharmaceutical formulations. Phosphate occurs extensively in the body and is involved in many physiological processes since it is the principal anion of intracellular fluid. Most foods contain adequate amounts of phosphate, making hypophosphatemia (phosphate deficiency) virtually unknown except for certain disease states or in patients receiving total parenteral nutrition. Treatment is usually by the oral administration of up to 100 mmol of phosphate daily.Approximately two-thirds of ingested phosphate is absorbed from the gastrointestinal tract, virtually all of it being excreted in the urine, and the remainder is excreted in the feces.
Excessive administration of phosphate, particularly intravenously, rectally, or in patients with renal failure, can cause hyperphosphatemia that may lead to hypocalcemia or other severe electrolyte imbalances. Adverse effects occur less frequently following oral consumption, although phosphates act as mild saline laxatives when administered orally or rectally. Consequently, gastrointestinal disturbances including diarrhea, nausea, and vomiting may occur following the use of dibasic sodium phosphate as an excipient in oral formulations. However, the level of dibasic sodium phosphate used as an excipient in a pharmaceutical formulation is not usually associated with adverse effects.
LD50 (rat, oral): 17 g/kg
저장
The anhydrous form of dibasic sodium phosphate is hygroscopic. When heated to 40℃, the dodecahydrate fuses; at 100℃ it loses its water of crystallization; and at a dull-red heat (about 240℃) it is converted into the pyrophosphate, Na4P2O7. Aqueous solutions of dibasic sodium phosphate are stable and may be sterilized by autoclaving.The bulk material should be stored in an airtight container, in a cool, dry place.
비 호환성
Dibasic sodium phosphate is incompatible with alkaloids, antipyrine, chloral hydrate, lead acetate, pyrogallol, resorcinol and calcium gluconate, and ciprofloxacin. Interaction between calcium and phosphate, leading to the formation of insoluble calcium-phosphate precipitates, is possible in parenteral admixtures.Regulatory Status
GRAS listed. Accepted in Europe for use as a food additive. Included in the FDA Inactive Ingredients Database (injections; infusions; nasal, ophthalmic, oral, otic, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.인산나트륨,이염기성,칠수화물 준비 용품 및 원자재
원자재
준비 용품
인산나트륨,이염기성,칠수화물 공급 업체
글로벌( 204)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 |
1056@dideu.com | China | 2878 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34571 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 9126 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
info@afinechem.com | China | 15396 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
인산나트륨,이염기성,칠수화물 관련 검색:
인산 나트륨, 이염기성, 도데카수화물 인산 나트륨, 이염기
MAGNESIUM HYDROGEN PHOSPHATE TRIHYDRATE
DICALCIUM PHOSPHATE DIHYDRATE
Ammonium phosphate dibasic
Tris(2-butoxyethyl) phosphate
Triethyl phosphate
Dipotassium hydrogen phosphate trihydrate
Tris(2-ethylhexyl) phosphate
Disodium hydrogen phosphate dihydrate
Triisobutyl phosphate
Triphenyl phosphate
2'-Deoxyguanosine-5'-diphosphate trisodium salt
Nicotinamide hypoxanthine dinucleotide phosphate reduced tetrasodium salt
CDP
IDP
iron sodium diphosphate
Nadph tetrasodium salt