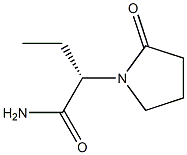
Levetiracetam C화학적 특성, 용도, 생산
개요
Levetiracetam was first introduced in the US as an adjunctive therapy in the treatment
of partial-onset seizures in adults with epilepsy. This second-generation analog of
piracetam can be prepared by condensation of (S)-2-aminobutyramide with 4-chlorobutyryl
chloride. Although its mechanism of action is not well established, it was shown that [
3H]-levetiracetam
reversibly binds to a specific site predominantly present in the membranes of
the brain. Unlike conventional anticonvulsants such as phenytoin, carbamazepine, valproic
acid, phenobarbital, diazepam and clonazepam, compounds structurally-related to
levetiracetam, such as piracetam and aniracetam, also have affinity for this site.
Levetiracetam reveals a broad and unique profile in animal seizure models, including
promising antiepileptogenic properties. Besides being rapidly and almost completely
absorbed in man (oral bioavailability>95%), it possesses a favorable pharmacokinetic
profile since it is not hepatically metabolized but only partly hydrolized into the inactive
carboxylic acid by enzymes in a number of tissues including blood cells, it is minimally
bound to plasma proteins (<10%) and does not inhibit or induce hepatic enzymes.
Therefore levetiracetam has a low potential for drug interaction, providing a useful
alternative as adjunctive therapy to treat seizures refractory to conventional
anticonvulsants.
화학적 성질
White Crystalline Solid
용도
The (S)-enantiomer of the ethyl analog of Piracetam. Used as an anticonvulsant.
정의
ChEBI: A pyrrolidinone and carboxamide that is N-methylpyrrolidin-2-one in which one of the methyl hydrogens is replaced by an aminocarbonyl group, while another is replaced by an ethyl group (the S enantiomer). An anticonvulsa
t, it is used for the treatment of epilepsy in both human and veterinary medicine.
Biological Functions
Levetiracetam (Keppra) has recently been approved for
the treatment of partial-onset seizures. It appears to be
safe and effective; its exact therapeutic profile has yet to
be determined. It does not appear to share any of the
mechanisms of action of agents that have been discussed
to this point. It does have a highly specific brain
binding site, but the significance of this observation to
its mechanism of action has not been elucidated.
일반 설명
LEV is an analog of the nootropic agent, piracetam. Onlythe S-isomer has any anticonvulsant activity. Unlike piracetam, LEV does not have any affinity for the AMPA receptor thereby has no nootropic activity forthe treatment of Alzheimer disease. LEV also has no affinityfor GABA receptors, BZD receptors, the various excitatoryamino acid related receptors, or the voltage-gated ionchannels.For this reason, its mechanism of anticonvulsantaction remains unclear, but it appears to exert itsantiepileptic action by modulating kainite/AMPA-inducedexcitatory synaptic currents, thus decreasing membraneconductance.Furthermore, the anticonvulsant activity ofthis drug appears to be mediated by the parent moleculerather than by its inactive metabolite,(S)-α-ethyl-2-oxo-1-pyrrolidineacetic acid (i.e., via the hydrolysis of amidegroup).Like gabapentin, LEV has few drug interactionswith other AEDs thereby can be used in combination to treatrefractory epilepsy.
생물학적 활성
Antiepileptic that displays distinctive properties from conventional antiepileptic drugs. Displays potent seizure protection in animal models of chronic epilepsy but lacks activity in acute seizure models. Binds synaptic vesicle protein 2A (SV2A) and inhibits Na + -dependent Cl - /HCO 3 - exchange.
Mechanism of action
The mechanism of action for S-(–)-levetiracetam is unknown. It does not appear to interact with any of the recognized
excitatory or inhibitory neural mechanism. A CNS-specific binding site for S-(–)-levetiracetam has been identified as the
synaptic vesicle protein (SV2A). Knockout animals without SV2A proteins accumulated presynaptic Ca2+ during consecutive
action potentials that destabilized synaptic circuits and induced epilepsy. Thus, it appears that SV2A plays a major role in the
antiepileptic properties of S-(–)-levetiracetam, which acts by modulating the function of SV2A and the regulation of
Ca2+ mediated synaptic transmission. These data support previous indications that S-(–)-levetiracetam possesses a mechanism
of action distinct from that of other antiepileptic drugs. Three SV2 isoforms (SV2A, SV2B, and SV2C) have been identified, each
of which has a unique distribution in brain, suggesting synapse-specific functions as well as antagonism of neuronal
synchronization.
Pharmacokinetics
S-(–)-levetiracetam displays rapid and complete absorption, although food slows the rate but not the extent of absorption. It exhibits linear pharmacokinetics and is minimally protein bound. Approximately 60% of an oral dose is excreted into the
urine unchanged and 24 to 30% as its carboxylic acid metabolite, with an elimination half-life in adults of approximately 7
hours. Although S-(–)-levetiracetam is not metabolized by hepatic CYP450, UGT, or epoxide hydrolase, it is esterase
hydrolyzed to its carboxylic acid metabolite (loss of amido group), which is not affected by the hepatic metabolizing enzymes.
Clinical Use
S-(–)-levetiracetam is a pyrrolidone derivative unrelated to the structures of other AEDs. It is indicated as an adjunct in the
treatment of partial onset seizures in adults, and it has shown some benefit in clinical trials for generalized tonic-clonic
seizures (GTC) and myoclonic seizures in adults and children.
부작용
The risk of clinically relevant drug interactions is minimal with S-(–)-levetiracetam, because it does not alter the
pharmacokinetics of coadministered drugs by inhibition or induction of hepatic enzymes. Toxic effects include mild to
moderate somnolence, asthenia, ataxia, and dizziness; these effects seldom require discontinuance. An increase in the
incidence of behavioral abnormalities in children and in adults having a previous history of neuropsychiatric problems has been
noted. Its use in the elderly or in patients with renal impairment will require an individualization of dose, and an additional
dose is needed after renal dialysis. Levetiracetam was associated with developmental toxicity in the offspring of pregnant
animals.
Levetiracetam 준비 용품 및 원자재
원자재
준비 용품