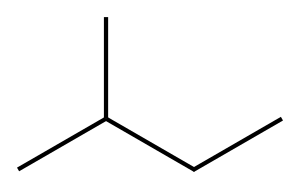
이소펜탄
|
|
이소펜탄 속성
- 녹는점
- -160 °C
- 끓는 점
- 30 °C(lit.)
- 밀도
- 0.62 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.6 (vs air)
- 증기압
- 11.17 psi ( 20 °C)
- 굴절률
- n
20/D 1.354(lit.)
- 인화점
- −51 °C
- 저장 조건
- 2-8°C
- 용해도
- 0.048g/L
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- >14 (Schwarzenbach et al., 1993)
- 색상
- 무색의
- 냄새
- 휘발유 같은 냄새
- 폭발한계
- 1.3-7.6%(V)
- Odor Threshold
- 1.3ppm
- 수용성
- 물, 탄화수소, 오일, 알코올 및 에테르와 혼합됩니다.
- 최대 파장(λmax)
- λ: 192 nm Amax: 1.00
λ: 210 nm Amax: 0.30
λ: 220 nm Amax: 0.07
λ: 240-400 nm Amax: 0.01
- BRN
- 1730723
- Henry's Law Constant
- (atm?m3/mol): 1.24 at 25 °C (approximate - calculated from water solubility and vapor pressure)
- 노출 한도
- ACGIH TLV: TWA 600 ppm (adopted).
- Dielectric constant
- 1.8(20℃)
- 안정성
- Stable. Extremely flammable. Incompatible with strong oxidizing agents. Vapour-air mixtures explosive in certain proportions. Incompatible with rubber, various plastics. The vapour, being heavier than air, may roll over surfaces and collect in low points. Note low flash point.
- LogP
- 3.4 at 20℃
- CAS 데이터베이스
- 78-78-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F+,Xn,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 12-51/53-65-66-67 | ||
| 안전지침서 | 9-16-29-33-61-62 | ||
| 유엔번호(UN No.) | UN 1265 3/PG 1 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | EK4430000 | ||
| 자연 발화 온도 | 788 °F | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | I | ||
| HS 번호 | 29011000 | ||
| 유해 물질 데이터 | 78-78-4(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-23537 |
이소펜탄 C화학적 특성, 용도, 생산
화학적 성질
2-Methylbutane (isopentane), C5H12, is a flammable liquid and exhibits physical properties very similar to those of pentane. It has been detected in urban air.
2-Methylbutane is an alkane that is butane substituted by a methyl group at position 2. It has a higher BP than butane because, although similarly branched, it has a higher MW. A useful analogy for comparing molecules with the same size of longest chain is that of cylindrical-shaped molecules. It has a role as a refrigerant. Biological samples flash frozen for example with a combination of liquid nitrogen and methylbutane can then be used for storage, cryosection, etc.
물리적 성질
Clear, colorless, watery, very flammable liquid with a pleasant odor. Evaporates quickly when spilled. An odor threshold concentration of 1.3 ppmv was reported by Nagata and Takeuchi (1990).용도
Isopentane is an organic, branched-chain alkane with five carbon atoms. 2-Methylbutane undergoes catalytic dehydrogenation in the presence of chromia-alumina catalyst to form isoamylenes, which can undergo further dehydrogenation to form isoprene. It may also be used as a solvent in the preparation of trans-Bis(triethylphosphine) (hydroxy carbonyl) (phenyl) platinum(II), a metallacarboxylic acid.생산 방법
Isopentane is produced by fractional distillation of natural gas liquids and crude oil.제조 방법
2-Methylbutane was synthesized by liquid-phase catalytic isomerization at 95°C using pentane as raw material.정의
ChEBI: 2-methylbutane is an alkane that is butane substituted by a methyl group at position 2. It has a role as a refrigerant.일반 설명
Watery colorless liquid with a gasoline-like odor. Floats on water. Flammable, irritating vapor is produced. Boiling point is 82°F.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
2-Methylbutane is a fire and explosion hazard when in contact with oxidizing agents. .건강위험
Inhalation causes irritation of respiratory tract, cough, mild depression, irregular heartbeat. Aspiration causes severe lung irritation, coughing, pulmonary edema; excitement followed by depression. Ingestion causes nausea, vomiting, swelling of abdomen, headache, depression.화재위험
Behavior in Fire: Highly volatile liquid. Vapors may explode when mixed with air.Safety Profile
Mddly toxic and narcotic by inhalation. See also PENTANE. Flammable liquid. A very dangerous fire and explosion hazard when exposed to heat, flame, or oxidzers. Keep away from sparks, heat, or open flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.환경귀착
Photolytic. When synthetic air containing gaseous nitrous acid and 2-methylbutane was exposed to artificial sunlight (λ = 300–450 nm), acetone, acetaldehyde, methyl nitrate, peroxy-acetal nitrate, propyl nitrate, and pentyl nitrate were formed (Cox et al., 1980).Based upon a photooxidation rate constant of 3.90 x 10-12 cm3/molecule?sec with OH radicals in summer daylight, the atmospheric lifetime is 36 h (Altshuller, 1991). At atmospheric pressure and 300 K, Darnall et al. (1978) reported a rate constant of 3.78 x 10-12 cm3/molecule?sec for the same reaction.
Cox et al. (1980) reported a rate constant of 5.0 x 10-11 cm3/molecule?sec for the reaction of gaseous 2-methylbutane with OH radicals based on a value of 8 x 10-12 cm3/molecule?sec for the reaction of ethylene with OH radicals.
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor.
2-Methylbutane will not hydrolyze because it does not contain a hydrolyzable functional group.
Purification Methods
Stir isopentane for several hours in the cold with conc H2SO4 (to remove olefinic impurities), then wash it with H2O, aqueous Na2CO3 and H2O again. Dry it with MgSO4 and fractionally distil it using a Todd column packed with glass helices. Material transparent down to 180nm is obtained by distilling from sodium wire, and passing through a column of silica gel which had previously been dried in place at 350o for 12hours before use. [Potts J Phys Chem 20 809 1952, Beilstein 1 IV 320.]이소펜탄 준비 용품 및 원자재
원자재
준비 용품
이소펜탄 공급 업체
글로벌( 300)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9020 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12470 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1534 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 15954 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21687 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8829 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |