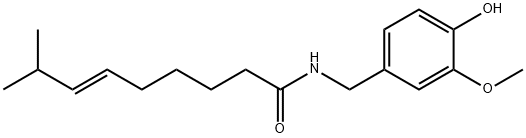
캡사이신 C화학적 특성, 용도, 생산
개요
Capsaicin has a mild, warm-herbaceous odor and a burning pungent taste (at 10 ppm). It is used in compounded flavors for sauces where the pungent note is desired. This substance is present in several species of Capsicum (Family, Solanaceae). The sensation of pain, accompanied by irritation and inflammation, is due to substance P depletion from sensory (afferent) nerve fibers. These properties are used to study the physiology of pain and the effects as a counterirritant and gastrointestinal stimulant. This substance may be prepared from 3-chloro-2-isopropyltetrahydropyran; biosynthesis from Capsicum frutescens; separation form cis-capsaicin, pelargonic acid vanilamide, and dihydrocapsaicin, reaction of capsaicin.
화학적 성질
Off-White Crystalline Solid
물리적 성질
Appearance: crystalline white powder, with highly volatile and pungent odor.
Solubility: freely soluble in alcohol, ether, benzene, and chloroform; slightly soluble
in carbon disulfide, petroleum, and hydrochloric acid; insoluble in water. Melting
point: 65?°C.
출처
The pungent principle in the fruits of various Capsicum species (Solanaceae)
용도
Capsaicin analogue (C175680). It is used as a tool in neurobiological research. Prototype vanilloid receptor agonist. Topical analgesic.
Indications
Capsaicin (Zostrix) is approved for the relief of pain
following herpes zoster infection (postherpetic neuralgia).
The drug depletes neurons of substance P, an endogenous
neuropeptide that may mediate cutaneous
pain. It is applied to affected skin after open lesions
have healed. Local irritation is common.
일반 설명
Capsaicin occurs as the active ingredient of hot/red pepper and was first obtained by Thresh in 1846. It is a lipophilic vanilloid compound responsible for the acrid taste of hot peppers.
생물학적 활성
Prototypic vanilloid receptor agonist (pEC 50 values are 7.97 and 7.10 at rat and human VR1 receptors respectively). Excites a subset of primary afferent sensory neurons, with subsequent antinociceptive and anti-inflammatory effects. Reversibly inhibits aggregation of platelets. Also available as part of the Vanilloid TRPV1 Receptor Tocriset™ .
Anticancer Research
Capsaicin is the major pungent ingredient in red and green chili pepper. It is reportedto induce apoptosis selectively in cancer cells and can suppress the activation ofNF-κB through suppression of NF-κB inhibitor IκBα (Aggarwal and Shishodia 2004). It shows anticancer effects in animal models and suppresses carcinogenesisin colon, skin, lung, tongue, and prostate cancers by altering the metabolism ofcarcinogens. It selectively suppresses the human cancer cell growth of prostate,leukemic, glioma, gastric, and hepatic cancers. It inhibited the tumorigenesis linkedand IL-6-induced activation of STAT-3 and STAT3-regulated gene products likecyclin D1, Bcl-2, Bcl-xL, survivin, and VGEF. It arrests cells in G1 phase andinduces apoptosis (Aggarwal et al. 2008; Clark and Lee 2016).
Clinical Use
In clinical practice, capsaicin is mainly used for topical administration, such as in
the treatment of osteoarthritis and rheumatoid arthritis pain, diabetic nerve pain,
pain after surgery, chemotherapy- or radiotherapy-induced oral pain, psoriasis, etc.
Capsaicin irritates the mucous membrane to cause sneezing, nose bleeding,
coughing, mucus secretion, tears, bronchoconstriction, breathing difficulties, and
other symptoms. The main adverse effects of capsaicin preparations are contact
dermatitis, skin inflammation or blisters, and in severe situation burn-like lesion.
잠재적 노출
Botanical animal and insect repellent
used to repel birds, voles, deer, rabbits, squirrels, insects,
and attacking dogs. Capsaicin, which is made from the
Capsicum red chili pepper can be used indoors to protect
carpets and upholstered furniture, and outdoors to protect
fruit and vegetable crops, flowers, ornamental plants,
shrubbery, trees, and lawns. It is also used in pepper sprays
such as MACE, and as an analgesic in creams, lotions and
solid sticks to reduce arthritic, postoperative and neuopathic
pain, such as shingles. Capsaicin is obtained by grinding
dried, ripe Capsicum frutescens L. chili peppers into a fine
powder. The oleoresin is derived by distilling the powder
in a solvent and evaporating the solvent. The resulting
highly concentrated liquid has little odor but has an
extremely pungent taste
운송 방법
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Purification Methods
Recrystallise capcaicin from pet ether (b 40-60o), or pet ether/Et2O (9:1). Also purify it by chromatography on neutral Al2O3 (grade V) and elute successively with *C6H6, *C6H6/EtOAc (17:3) then *C6H6/EtOAc (7:3), and distil it at 120o/10-5mm, then repeatedly recrystallise the needles from isopropanol (charcoal). [Crombie et al. J Chem Soc 11025 1955, Bennett & Kirby J Chem Soc(C) 442 1968.] It causes pain and is neurotoxic [Bevan & Szolcsanyi Trends in Pharmacol Sci 11 330 1990, Beilstein 13 IV 2588].
비 호환성
Slowly hydrolyzes in water, releasing
ammonia and forming acetate salts.
폐기물 처리
Do not discharge into drains
or sewers. Dispose of waste material as hazardous waste
using a licensed disposal contractor to an approved landfill.
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Incineration with
effluent gas scrubbing is recommended. In accordance
with 40CFR165, follow recommendations for the disposal
of pesticides and pesticide containers. Noncombustible containers should be crushed and buried under more than
40 cm of soil. Must be disposed properly by following
package label directions or by contacting your local or federal environmental control agency, or by contacting your
regional EPA office
캡사이신 준비 용품 및 원자재
원자재
준비 용품