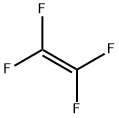
테트라플루오르에틸렌
|
|
테트라플루오르에틸렌 속성
- 녹는점
- -142°C
- 끓는 점
- -76.3°C
- 밀도
- 1.1507
- 굴절률
- 1.2420 (estimate)
- 수용성
- 110mg/L at 28℃
- Dielectric constant
- 1.9(Ambient)
- 안정성
- 안정적인. 가열되면 반응합니다.
- LogP
- 1.21 at 20℃
- CAS 데이터베이스
- 116-14-3(CAS DataBase Reference)
- IARC
- 2A (Vol. 19, Sup 7, 71, 110) 2017
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11 | ||
| 안전지침서 | 16-36/37/39-45 | ||
| 유엔번호(UN No.) | 1081 | ||
| 위험 참고 사항 | Corrosive/Flammable | ||
| 위험 등급 | 2.1 | ||
| 유해 물질 데이터 | 116-14-3(Hazardous Substances Data) | ||
| 독성 | LC50 (4 hr) in rats, mice, hamsters, guinea pigs (ppm): 37500-45000, 35000, 28500, 28000 (Kennedy) | ||
| 기존화학 물질 | KE-33427 | ||
| 중점관리물질 필터링 | 별표1-68 | ||
| 암, 돌연변이성물질 필터링 | 342 | ||
| 사고대비 물질 필터링 | 72 |
테트라플루오르에틸렌 C화학적 특성, 용도, 생산
개요
Tetrafluoroethylene is a synthetic, colorless, flammable gas that is insoluble in water. Tetrafluoroethylene is used primarily in the synthesis of polytetrafluoroethylene resins. It is also used as a monomer in the synthesis of copolymers and as a propellant for food product aerosols. When heated to decomposition, tetrafluoroethylene emits highly toxic fluorocarbon fumes. The primary route of human exposure to this compound is inhalation. Acute inhalation exposure to tetrafluoroethylene may result in irritation of the respiratory tract and buildup of fluid in the lungs (pulmonary edema). Contact with this gas can cause eye irritation. This chemical is reasonably anticipated to be a human carcinogen. (NCI05)화학적 성질
Tetrafluoroethylene is a colorless, flammable gas. Heavier than air. insoluble in water. soluble in acetone.용도
In manufacture of polymers and synthesis of fluorinated refrigerants, dielectric media and solvents. In vinyl polymerization, cycloalkylation and addition reactions.제조 방법
Tetrafluoroethylene (TFE) is manufactured from chloroform. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane then yields TFE.CHCl3 + 2 HF → CHClF2 + 2 HCl
2CHClF2 → C2F4 + 2 HCl
A laboratory synthesis entails pyrolysis of a PTFE under a vacuum. The PTFE polymer "cracks" and depending on the pressure, produces mainly C2F4.
주요 응용
The main use of tetrafluoroethylene is in the manufacture of polytetrafluoroethylene (PTFE) that is used as nonstick coatings on cookware, membranes for clothing that are both waterproof and breathable, electrical-wire casing, fire- and chemical-resistant tubing, and plumbing thread seal tape.The most widely known PTFE formulation is sold under the brand name of Teflon®. PTFE was discovered by DuPont Co. in 1938.정의
Tetrafluoroethene is a fluorocarbon. It is a gaseous organic compound (a fluorocarbon and a haloalkene) used to make the plastic polytetrafluoroethene (PTFE).일반 설명
Tetrafluoroethylene, stabilized appears as a colorless odorless gas. Easily ignited. Vapors are heavier than air. May asphyxiate by the displacement of air. May violently polymerize under prolonged exposure to fire or heat, violently rupturing the container. Under prolonged exposure to fire or heat the containers may rupture violently and rocket. Water insoluble.공기와 물의 반응
Flammable. Forms polymeric peroxides that are explosive [Bretherick 1979 p. 164].반응 프로필
Tetrafluoroethylene reacts with air (oxygen) to form polymeric peroxides that are explosive [Bretherick 1979 p. 164]. Probably susceptible to similar reactions with a number of oxidizing agents.May polymerize violently (inhibitor tends to prevent this reaction). May react violently with aluminum. Contamination of a tetrafluoroethylene gas supply system led to a reaction between the inhibitor, limonene, and the contaminant, iodine pentafluoride. This initiated an explosive polymerization event [MCA Case History No. 1520].위험도
Flammable, dangerous fire risk. Kidney and liver damage; kidney and liver cancer. Possible carcinogen.건강위험
Inhalation causes irritation of respiratory system. Contact with eyes causes slight irritation.Safety Profile
Confirmed carcinogen. Mildly toxic by inhalation. Can act as an asphyxiant and may have other toxic properties. The gas is flammable when exposed to heat or flame. The inhibited monomer will explode if igntted. Explosive in the form of vapor when exposed to heat or flame. Will explode at pressures above 2.7 bar if limonene inhbitor is not added. Iodine pentafluoride depletes the limonene inhbitor and then causes explosive polymerization of the monomer. Mixtures with hexafluoropropene and air form an explosive peroxide. Reacts violently with SO3; air; dfluoromethylene dihypofluorite; loxygen difluoride; iodine pentafluoride; oxygen. When heated to decomposition it emits highly toxic fumes of F-. See also FLUORIDES.잠재적 노출
A potential danger to those involved in the production of TFE and the manufacture of fluorocarbon polymers.Carcinogenicity
Tetrafluoroethylene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals (NTP 1997).비 호환성
Reacts with air. Hazardous polymerization may occur unless inhibited. Will explode at pressures above 2.7 bar if terpene inhibitor is not added. Inhibited monomer can decompose explosively in fire, under pressure, or upon contact with materials with which it can react exothermically. Violent reaction with oxygen, oxidizers, sulfur trioxide; halogen compounds.폐기물 처리
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillable-type cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.테트라플루오르에틸렌 준비 용품 및 원자재
원자재
LACQUER THINNER
클로로디플루오르메탄
옥타플루오로사이클로부탄
1-클로로-1,1,2,2,3,3-헥사플루오로프로판
퍼플루오로아이소뷰틸렌
다이클로로모노플루오로메테인(디클로로모노플루오로메탄)
준비 용품
헥사플루오로아세톤
potassium ω-hydroperfluroheptylate
폴리테트라플루오르에틸렌
1,2-다이브로모테트라플루오로에탄
perfluorinated sulfonic acid resin
perfluoroalkylether potassium sulfonate
3,5-Dichloro-4-(1,1,2,2-tetrafluoroethoxy)aniline
헥사플루오로프로필렌
테트라플루오로옥시란
2,2,3,3,3-펜타플루오로프로필메틸에테르
1,3-디브로모헥사플루오로프로판
1,4-디브로모옥타플루오로부탄
1H,1H,9H-헥사데카플루오로-1-노난올
테트라플루오르에틸렌 공급 업체
글로벌( 44)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21687 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Hebei Duling International Trade Co. LTD | +8617333973358 |
sales01@hbduling.cn | China | 15620 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23931 | 58 |
테트라플루오르에틸렌 관련 검색:
폴리테트라플루오르에틸렌 테트라코나졸 테트라플루오르에틸렌-헥사플루오르프로필렌 공중합체 테트라플루오르에텐, 에텐과의 중합체 트라이플루오로(트라이플루오로메톡시)에텐을 포함한 테트라플루오로에텐 중합체 1,1,1,2,2,3,3-헵타플루오르-3-((트리플루오르에텐일)옥시)프로판, 테트라플루오르에텐과의 중합체 테트라플루오르에텐, 산화된, 중합체 비닐 플루오르화물 플루오르화비닐리덴 테트라플루오르에틸렌 알파-클로로-오메가-(2,2-디클로로-1,1,2-트리플루오르에틸)-폴리 (디플루오르메틸렌)
2,3-[(Tetrafluoroethylene)dioxy]aniline
TRIFLUOROETHYLENE
4-Bromo-1,2-[(tetrafluoroethylene)dioxy]benzene~3,4-[(Tetrafluoroethylene)dioxy]bromobenzene
3,4-[(Tetrafluoroethylene)dioxy]aniline
Tetrafluoroethylene diiodide
1,1'-[(1,1,2,2-tetrafluoroethylene)bis(oxy)]bis[1,2,2-trifluoroethylene]
1,2-DIFLUOROETHYLENE