Eptifibatide Acetate
|
|
Eptifibatide Acetate 속성
- 녹는점
- >220°C (dec.)
- 저장 조건
- Refrigerator
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨), 물(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 황백색까지
- CAS 데이터베이스
- 148031-34-9(CAS DataBase Reference)
안전
Eptifibatide Acetate C화학적 특성, 용도, 생산
Indications
Acute Coronary Syndrome (ACS)INTEGRILIN? is indicated to decrease the rate of a combined endpoint of death or new myocardial infarction (MI) in patients with ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI).
Percutaneous Coronary Intervention (PCI)
INTEGRILIN is indicated to decrease the rate of a combined endpoint of death, new MI, or need for urgent intervention in patients undergoing PCI, including those undergoing intracoronary stenting.
주요 응용
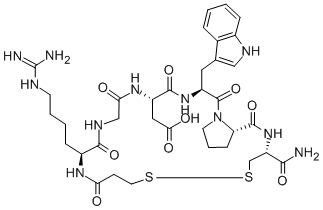
Eptifibatide is a cyclic heptapeptide containing 6 amino acids and 1 mercaptopropionyl (des-amino cysteinyl) residue. An interchain disulfide bridge is formed between the cysteine amide and the mercaptopropionyl moieties. Chemically it is N6-(aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl)-Llysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic (1→6)-disulfide. Eptifibatide binds to the platelet receptor glycoprotein (GP) IIb/IIIa of human platelets and inhibits platelet aggregation.The eptifibatide peptide is produced by solution-phase peptide synthesis, and is purified by preparative reverse-phase liquid chromatography and lyophilized. The structural formula is:

Dosage forms
Before infusion of INTEGRILIN, the following laboratory tests should be performed to identify pre-existing hemostatic abnormalities: hematocrit or hemoglobin, platelet count, serum creatinine, and PT/aPTT. In patients undergoing PCI, the activated clotting time (ACT) should also be measured.The activated partial thromboplastin time (aPTT) should be maintained between 50 and 70 seconds unless PCI is to be performed. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT and ACT.
Eptifibatide Acetate 준비 용품 및 원자재
원자재
준비 용품
Eptifibatide Acetate 공급 업체
글로벌( 289)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Cellmano Biotech Limited | 0551-65326643 18156095617 |
info@cellmano.com | China | 999 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 18958018566; |
info@afinechem.com | China | 15377 | 58 |
| Chengdu Youngshe Chemical Co., Ltd. | +8618108235634 |
Cecilia@youngshechem.com | China | 2345 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 992 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 |
Mandy@hangyubiotech.com | China | 11013 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 |
admin@guyunchem.com | China | 616 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 1142 | 58 |
| Alpha Biopharmaceuticals Co., Ltd | +86-411-39042497 +8613921981412 |
sales@alphabiopharm.com | China | 886 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9341 | 55 |