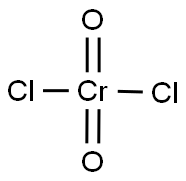
크로밀 염화물
|
|
크로밀 염화물 속성
- 녹는점
- -96.5 °C (lit.)
- 끓는 점
- 117 °C (lit.)
- 밀도
- 1.911 g/mL at 25 °C (lit.)
- 인화점
- 117°C
- 용해도
- reacts with H2O; soluble in ctc, chloroform,benzene
- 물리적 상태
- 액체
- Specific Gravity
- 1.911
- 색상
- 빨간색
- 수용성
- 사염화탄소, 이황화탄소, 벤젠, 니트로벤젠, 클로로포름 및 POCL3에 용해됩니다. 물에 불용성(반응하다).
- 감도
- Moisture Sensitive
- Merck
- 14,2248
- Dielectric constant
- 2.6(20℃)
- 노출 한도
- ACGIH: TWA 0.0001 ppm; STEL 0.00025 ppm (Skin)
NIOSH: TWA 0.0002 mg/m3
- CAS 데이터베이스
- 14977-61-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,T,C,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 49-46-8-35-43-50/53-34-23/24/25-45 | ||
| 안전지침서 | 53-45-60-61-36/37/39-27-26-17 | ||
| 유엔번호(UN No.) | UN 3244 8/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | GB5775000 | ||
| F 고인화성물질 | 8-10-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | I | ||
| HS 번호 | 2827499090 | ||
| 유해 물질 데이터 | 14977-61-8(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-06024 | ||
| 유해화학물질 필터링 | 06-5-10 | ||
| 중점관리물질 필터링 | 별표2-298 | ||
| 암, 돌연변이성물질 필터링 | 190 | ||
| 함량 및 규제정보 | 물질구분: 제한물질; 혼합물(제품)함량정보: 크로뮴(6+)화합물 및 이를 0.1% 이상 함유한 혼합물 |
크로밀 염화물 C화학적 특성, 용도, 생산
화학적 성질
Chromyl chloride is a dark red fuming liquid with a musty, burning odor.물리적 성질
Dark red, fuming liquid; reddish yellow vapors; musty buring odor; density 1.91 g/mL; freezes at -96.5°C; boils at 117°C; reacts with water; soluble in chloroform, carbon tetrachloride, benzene, carbon disulfide and nitrobenzene.용도
Highly spin-polarized chromium dioxide (CrO2) thin films were deposited on 100 TiO2 substrates by chemical vapor deposition using chromyl chloride as a precursor. Chromyl chloride (Cr02C12) reacts with cyclohexane solvent at 75 OC to give a dark precipitate along with chlorocyclohexane and a small amount of cyclohexene (in 10.0 and ca. 0.3% yields based on chromium). Chromyl chloride, CrO2C12, oxidizes cyclooctane, isobutane, and toluene under mild conditions (25-60" C). The reactions give chlorinated products (chlorocyclooctane, tert- butyl chloride, and benzyl chloride) and a dark chromium-containing precipitate. Used for Organic oxidations and chlorination, chromium coordination complexes, catalyst for polymerization of olefins. Chromyl chloride in carbon tetrachloride solution reacts in the cold with cyclohexene, cyclopentene, and 1-hexene to give the various isomeric chlorohydrins as the major products.제조 방법
Chromyl chloride is prepared by reacting chromium(III) chloride with hydrochloric acid:CrO3 + 2HCl → CrO2Cl2 + H2O
Also, it may be prepared by warming potassium dichromate with potassium chloride in concentrated sulfuric acid:
K2Cr2O7 + 4KCl + 3H2SO4 → 2Cr2O2Cl2 + 3K2SO4 + 3H2O.
정의
A dark red covalent liquid prepared either by distilling a dry mixture of potassium dichromate and sodium chloride with concentrated sulfuric acid or by the action of concentrated sulfuric acid on chromium(VI) oxide dissolved in concentrated hydrochloric acid. Chromyl chloride is hydrolyzed by water, and with solutions of alkalis it undergoes immediate hydrolysis to produce chromate ions. It is used as an oxidizing agent in organic chemistry. Chromyl chloride oxidizes methyl groups at the ends of aromatic side chains to aldehyde groupings (étard’s reaction).일반 설명
A dark red fuming liquid with a pungent odor. Corrosive to tissue.반응 프로필
Chromyl chloride is a powerful and often violent oxidizing agent. Reacts readily with many inorganic and organic materials in the absence of a dilutent. Contact with hydrogen sulfide or phosphine can cause ignition. Contact with phosphorus tribromide, acetone, ethanol, ether, and turpentine causes ignition. Contact with moist phosphorus or with phosphorus trichloride leads to explosive reaction. Contact with ammonia causes incandescence. Reacts with sodium azide to form chromyl azide, which is explosive in the absence of a dilutent. Causes ignition of flowers of sulfur and of urea on contact. [Bretherick, 1979, p. 822-823].위험도
Corrosive to tissue. Strong oxidizing agent. Skin and upper respiratory tract irritant. Probable carcinogen.건강위험
Inhalation causes severe irritation of upper respiratory system. Contact with eyes or skin causes irritation and burning. Ingestion causes burning of mouth and stomach.화재위험
Behavior in Fire: Vapors are very irritating to eyes and mucous membranes. May increase severity of fire.Safety Profile
Suspected carcinogen. Probably a poison by various routes. Mutation data reported. Corrosive. A strong irritant. Hydrolyzes to form chromic and hydrochloric acids. A strong oxidner and chlorinating agent. Violent reaction with water. Reacts violently with alcohol, ether, acetone, turpentine. Ignites or explodes on contact with nonmetal halides (e.g., disulfur dichloride, phosphorus trichloride, and phosphorus tribromide), nonmetal hydrides (e.g., hydrogen sulfide and hydrogen phosphide), flowers of sulfur, moist phosphorus, sodium azide, and urea. During preparation can violently explode. Incompatible with ammonia, disulfur dichloride, organic solvents, phosphorus, phosphorus trichloride, sodium azide, and sulfur. When heated to decomposition it emits toxic fumes of Cl-. See also CHROMIUM COMPOUNDS.잠재적 노출
Chromium oxychloride is used in making chromium complexes and dyes; and used in various organic oxidation and chlorination reactions운송 방법
UN1758 Chromium oxychloride, Hazard class: 8; Labels: 8-Corrosive material.Purification Methods
Purify it by distillation under reduced pressure. It hydrolyses violently with H2O and is a powerful oxidant which explodes with P, and ignites in contact with S, NH3, EtOH and many organic compounds. TOXIC.비 호환성
Contact with water is violent and forms hydrochloric and chromic acids, and chlorine gas. A powerful oxidizer. Reacts violently with acetone, alcohol, ammonia, ether, fuels, organic solvents, moist phosphorus, phosphorus trichloride; sodium azide; sulfur, reducing agents; turpentine. Contact with nonmetal halides, such as disulfur dichloride, phosphorus trichloride; and phosphorus tribromide; nonmetal hydrides, such as hydrogen sulfide; hydrogen phosphide, and urea, causes a danger fire and explosion hazard크로밀 염화물 준비 용품 및 원자재
원자재
준비 용품
크로밀 염화물 공급 업체
글로벌( 48)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 |
1056@dideu.com | China | 3131 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| Alfa Chemistry | |
info@alfa-chemistry.com | United States | 2344 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9020 | 58 |
| Aladdin Scientific | +1-833-552-7181 |
sales@aladdinsci.com | United States | 52927 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 |
market03@meryer.com | China | 40240 | 62 |
| Alfa Aesar | 400-6106006 |
saleschina@alfa-asia.com | China | 30132 | 84 |