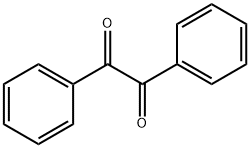
벤질
|
|
벤질 속성
- 녹는점
- 94-95 °C(lit.)
- 끓는 점
- 346 °C
- 밀도
- 1,521 g/cm3
- 증기압
- 1 mm Hg ( 128.4 °C)
- 굴절률
- 1.5681 (estimate)
- 인화점
- 346-348°C
- 저장 조건
- Store below +30°C.
- 용해도
- <0.5g/L
- 물리적 상태
- 결정성 분말
- 색상
- 하얀색
- 수용성
- 0.5g/L(20℃)
- Merck
- 14,1078
- BRN
- 608047
- Dielectric constant
- 13.0(94℃)
- 안정성
- 안정적인. 타기 쉬운. 강한 산화제와 호환되지 않습니다.
- InChIKey
- WURBFLDFSFBTLW-UHFFFAOYSA-N
- CAS 데이터베이스
- 134-81-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-37/39 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | DD1925000 | ||
| F 고인화성물질 | 10 | ||
| 위험 참고 사항 | Irritant | ||
| TSCA | Yes | ||
| HS 번호 | 29143900 | ||
| 독성 | LD50 orally in Rabbit: 2710 mg/kg | ||
| 기존화학 물질 | KE-12065 |
벤질 C화학적 특성, 용도, 생산
화학적 성질
yellow crystals or powder용도
Benzil is used as a pharmaceutical intermediate and uv curing resin photosensitizer. In polymer chemistry, it is used as a photoinitiator. Further, it serves as a potent inhibitor of human carboxylesterases. It is used in the preparation of diketimines by reacting with amines. It is also involved in the famous benil-benzilic acid rearrangement. In addition this, it reacts with 1,3-diphenylacetone to get tetraphenylcyclopentadienone.정의
A reaction in which benzil (1,2- diphenylethan-1,2-dione) is treated with hydroxide and then with acid to give benzilic acid (2-hydroxy-2,2-diphenylethanoic acid):C6H5.CO.CO.C6H5→
(C6H5)2C(OH).COOH
The reaction, which involves migration of a phenyl group (C6H5-) from one carbon atom to another, was the first rearrangement reaction to be described (by Justus von Liebig in 1828).
benefits
Benzil has potential applications in biological metabolism and clinical medicine. Benzil derivates exhibit radical scavenging and antibacterial and hypertensive, antiprotozoal, antiproliferative, and antimitotic activities. Benzil derivatives have versatile applications in the pharmaceutical industry, and various heterocyclic compounds such as triazine, quinoxaline, and imidazole can be synthesized from 1,2-diketones. It is also used as a nanocatalyst in the application of imidazole derivatives. Recently, it is reported that benzil derivatives acted as photosensitive agents and photoinitiators due to its antitumor activity[1].Safety Profile
Low toxicity by ingestion. An eyeirritant. Combustible. When heated to decomposition itemits acrid smoke and irritating fumes.Safety
Potential Acute Health Effects: Very hazardous in case of skin contact (irritant), of eye contact (irritant). Hazardous in case of ingestion, of inhalation.The substance is toxic to lungs, mucous membranes. Repeated or prolonged exposure to the substance can produce target organs damage. Eye Contact: Check for and remove any contact lenses.
Fine dust dispersed in air may ignite. Dust can form an explosive mixture in air. Thermal decomposition can lead to release of irritating gases and vapors. Keep product and empty container away from heat and sources of ignition.
Purification Methods
Crystallise benzil from *benzene after washing with alkali. (Crystallisation from EtOH did not free benzil from material reacting with alkali.) [Hine & Howarth J Am Chem Soc 80 2274 1958.] It has also been crystallised from CCl4, diethyl ether or EtOH [Inoue et al. J Chem Soc, Faraday Trans 1 82 523 1986]. [Beilstein 7 IV 2502.]벤질 준비 용품 및 원자재
원자재
준비 용품
벤질 공급 업체
글로벌( 549)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Anhui Royal Chemical Co., Ltd. | +86-25-86655873 +8613962173137 |
marketing@royal-chem.com | China | 166 | 55 |
| Tianjin Huiren Chemtech Co., Ltd | +86-86-22-23707858 +86-86-13672008665 |
huirentech@foxmail.com | China | 169 | 58 |
| Ningxia Jinhua Chemical Co.,Ltd | 025-52279164 |
info@nxjhchem.com | China | 79 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12470 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 6011 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 |
sales03@chemcn.cn | China | 952 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 |
sales@frappschem.com | China | 885 | 50 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9341 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |