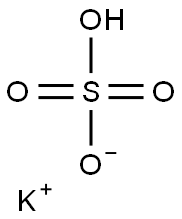
황산 칼륨
|
|
황산 칼륨 속성
- 녹는점
- 214 °C(lit.)
- 끓는 점
- decomposes [STR93]
- 밀도
- 2.32 g/mL at 25 °C(lit.)
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- Specific Gravity
- 2.322
- 수소이온지수(pH)
- 1 (50g/l, H2O, 20℃)
- 수용성
- 녹는
- 감도
- Hygroscopic
- Merck
- 14,7613
- 안정성
- 안정적인. 수분에 민감합니다.
- CAS 데이터베이스
- 7646-93-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-37 | ||
| 안전지침서 | 26-36/37/39-45 | ||
| 유엔번호(UN No.) | UN 2509 8/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TS7200000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| HS 번호 | 2833 29 80 | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| 독성 | LD50 orally in Rabbit: 2340 mg/kg | ||
| 기존화학 물질 | KE-32642 |
황산 칼륨 C화학적 특성, 용도, 생산
화학적 성질
Potassium bisulfate, also known as potassium hydrogen sulfate and potassium acid sulfate, KHS04 is water soluble, white deliquescent crystals,which melt at 214°C. Potassium bisulfate dissolved in water undergoes ionization to form K+, H+, SO42-. It is used in wine making, fertilizer manufacture, and as a flux and food preservative.주요 응용
Potassium bisulfate may be used:As a catalyst for the preparation of butyl paraben from p-hydroxybenzoic acid and n-butyl alcohol.
As a promoter for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via Biginelli reaction in ethylene glycol.
As an acid catalyst for the synthesis of ethyl tert-butyl ether (ETBE) from ethanol and tert-butyl alcohol via reactive distillation.
As a dehydrating agent for the synthesis of pyruvic acid from tartaric acid.
제조 방법
Potassium bisulfate is typically made by heating potassium sulfate (K2SO4) with sulfuric acid. The acid provides the hydrogen needed to convert the salt (K2SO4) to the corresponding acid salt (KHSO4).일반 설명
Potassium hydrogen sulfate is a colorless crystalline solid with a sulfur odor. It may cause illness from ingestion. If heated to high temperatures it may emit toxic fumes. It is used as flux in analysis of ores and siliceous Compounds.공기와 물의 반응
Fused salt is deliquescent, Soluble in water, yielding a corrosive acidic solution.반응 프로필
Acidic salts, such as Potassium bisulfate , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.건강위험
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Safety Profile
Moderately toxic by ingestion. A corrosive irritant to the skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of SOx and K2O. Can form an explosive mixture. See also SULFATES.Purification Methods
Crystallise it from H2O (1mL/g) between 100o and 0o. It is also formed when a warm solution of K2SO4 in conc H2SO4 is cooled down.황산 칼륨 준비 용품 및 원자재
원자재
준비 용품
1-BROMO-4-(METHYLSULPHONYL)-2-NITROBENZENE
4-Chloro-alpha-methylstyrene
4-METHYLUMBELLIFERYL PHOSPHATE DISODIUM SALT
2-Fluoropyridine-5-carboxaldehyde
8-Hydroxyquinoline potassium sulfate
락톤
1-BOC-4-ETHYL-4-PIPERIDINECARBOXYLIC ACID
아크롤레인
tert-Butyl N-(2-bromoethyl)carbamate
4-Vinylphenol
Boc-Lys(Boc)-OH
피루브 산, 나트륨 염
Cefpodoxime proxetil
칼륨 메타비아황산염
피루브산
N-Boc-D-proline
Ethyl N-Boc-4-methylpiperidine-4-carboxylate
2-SULFANYLISONICOTINIC ACID
Tirofiban
L-메티오닌
9-비닐안트라센
클로로(3-)시티렌
4-에텐일페놀 아세트산
황산 칼륨 공급 업체
글로벌( 244)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 18229 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12471 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 6000 | 58 |
| Jiangsu Kolod Food Ingredients Co.,Ltd. | +86-518-85110578 +8618805133257 |
sales3257@jskolod.com | China | 132 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21689 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |