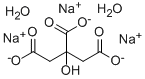
구연산 나트륨
|
|
구연산 나트륨 속성
- 녹는점
- >300 °C(lit.)
- 밀도
- 1.76
- 인화점
- 173.9 °C
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- H2O: 100 mg/mL
- 물리적 상태
- 가루
- 색상
- 하얀색
- 냄새
- 냄새 없는
- pH 범위
- 7.5 - 9 at 29.4 g/l at 25 °C
- 수소이온지수(pH)
- 7.0-9.0 (25℃, 50mg/mL in H2O)
- 수용성
- 720g/L(25℃)
- 최대 파장(λmax)
- λ: 260 nm Amax: 0.01
λ: 280 nm Amax: 0.01
- Merck
- 14,8602
- BRN
- 6104939
- 안정성
- 안정적인. 염기, 환원제, 산화제와 호환되지 않습니다.
- InChIKey
- NLJMYIDDQXHKNR-UHFFFAOYSA-K
- LogP
- -1.72
- CAS 데이터베이스
- 6132-04-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 37/38-41-36/37/38 | ||
| 안전지침서 | 26-36/37/39-24/25-36 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | GE7810000 | ||
| TSCA | Yes | ||
| HS 번호 | 2918 15 00 | ||
| 독성 | LD50 orally in Rabbit: > 8000 mg/kg |
구연산 나트륨 C화학적 특성, 용도, 생산
화학적 성질
white powder or colourless crystals용도
Trisodium citrate dihydrate, is widely applied in food, beverages and fillers as a buffering, sequestering or an emulsifying agent. It used as an anticoagulant in blood transfusions, osmotic laxative, functional fluids, solvents cleaning, furnishing care products, laundry dishwashing products and cleaning automobile radiators.생산 방법
Sodium citrate is prepared by adding sodium carbonate to a solution of citric acid until effervescence ceases. The resulting solution is filtered and evaporated to dryness.정의
ChEBI: The dihydrate of trisodium citrate.일반 설명
Sodium citrate tribasic dihydrate is the tribasic dihydrate sodium salt of citric acid.Pharmaceutical Applications
Sodium citrate, as either the dihydrate or anhydrous material, is widely used in pharmaceutical formulations.It is used in food products, primarily to adjust the pH of solutions. It is also used as a sequestering agent. The anhydrous material is used in effervescent tablet formulations. Sodium citrate is additionally used as a blood anticoagulant either alone or in combination with other citrates such as disodium hydrogen citrate.
Therapeutically, sodium citrate is used to relieve the painful irritation caused by cystitis, and also to treat dehydration and acidosis due to diarrhea.
생물학적 활성
Commonly used laboratory reagentSafety
After ingestion, sodium citrate is absorbed and metabolized to bicarbonate. Although it is generally regarded as a nontoxic and nonirritant excipient, excessive consumption may cause gastrointestinal discomfort or diarrhea. Therapeutically, in adults, up to 15 g daily of sodium citrate dihydrate may be administered orally, in divided doses, as an aqueous solution to relieve the painful irritation caused by cystitis.Citrates and citric acid enhance intestinal aluminum absorption in renal patients, which may lead to increased, harmful serum aluminum levels. It has therefore been suggested that patients with renal failure taking aluminum compounds to control phosphate absorption should not be prescribed citrate- or citric acid-containing products.
저장
Sodium citrate dihydrate is a stable material. Aqueous solutions may be sterilized by autoclaving. On storage, aqueous solutions may cause the separation of small, solid particles from glass containers.The bulk material should be stored in an airtight container in a cool, dry place.
Purification Methods
Crystallise the salt from warm water by cooling to 0o. [Beilstein 3 III 1100, 3 IV 1274.]비 호환성
Aqueous solutions are slightly alkaline and will react with acidic substances. Alkaloidal salts may be precipitated from their aqueous or hydro-alcohol solutions. Calcium and strontium salts will cause precipitation of the corresponding citrates. Other incompatibilities include bases, reducing agents, and oxidizing agents.Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (inhalations; injections; ophthalmic products; oral solutions, suspensions, syrups and tablets; nasal, otic, rectal, topical, transdermal, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.구연산 나트륨 준비 용품 및 원자재
원자재
준비 용품
구연산 나트륨 공급 업체
글로벌( 605)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12471 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 |
admin@china-yime.com | China | 563 | 58 |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5961 | 58 |
| Wuhan Han Sheng New Material Technology Co.,Ltd | +8617798174412 |
admin01@hsnm.com.cn | China | 2118 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 |
kyra@quanjinci.com | China | 1534 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 938 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 3001 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 2028 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18032476855 +86-18032476855 |
admin@hblongbang.com | China | 609 | 58 |
구연산 나트륨 관련 검색:
시트르산(구연산) 칼슘시트레이트 구연산 나트륨, 무수 아세트산 칼륨 시트르산, 무수물 모노염기성의 나트륨 시틀산염 시클로프로판카복산에틸에스테르 트리메틸롤프로판 엽산 시트르산 나트륨 디수소 시트레이트 트리-나트륨 시트르산염 나트륨 글루타르산염 구연산 나트륨
Ethyl 4-hydroxybenzoate,sodium salt
Sildenafil citrate
CITRIC ACID TRISODIUM SALT HYDRATE, DNASE, RNASE AND PROTEASE FREE, FOR MOLECULAR BIOLOGY, 99.8%
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, 1,2-dimethyl ester