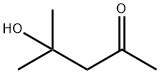
디아세톤알코올 C화학적 특성, 용도, 생산
용도
디 아세톤 알콜은 잘 알려진 합성 중간체이며 코팅, 페인트, 청소 제품 또는 농약과 같은 산업, 전문 및 소비자 용 제품의 많은 구성 요소로 사용됩니다.
화학적 성질
Diacetone alcohol is a colorless liquid. Mild,
mint odor.
물리적 성질
Clear, watery, flammable liquid with a mild, pleasant, characteristic odor similar to 2-butanone or
the pentanones. Experimentally determined detection and recognition odor threshold concentrations
were 1.3 mg/m
3 (270 ppb
v) and 5.2 mg/m
3 (1.1 ppm
v), respectively (Hellman and Small,
1974).
용도
Solvent for cellulose acetate, nitrocellulose, celluloid, fats, oils, waxes, resins. As a preservative in pharmaceutical preparations. In some antifreeze solutions and in hydraulic fluids.
생산 방법
4-Hydroxy-4-methyl-2-pentanone is manufactured through
the action of barium hydroxide, potassium hydroxide, or calcium
hydroxide on acetone. Commercial materials may contain
up to 15%acetone.
정의
ChEBI: A beta-hydroxy ketone formed by hydroxylation of 4-methylpentan-2-one at the 4-position. It has been isolated from Achnatherum robustum.
일반 설명
A clear colorless liquid with a pleasant odor. Flash point below 141°F. Less dense than water. Vapors heavier than air.
공기와 물의 반응
Highly flammable. Soluble in water.
반응 프로필
Acetyl bromide reacts violently with alcohols or water, [Merck 11th ed., 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: An explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid, [Chem. Eng. News 45(43):73(1967); J, Org. Chem. 28:1893(1963)]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites, [NFPA 491 M, 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)].
건강위험
Vapor is irritating to the mucous membrane of the eye and respiratory tract. Inhalation can cause dizziness, nausea, some anesthesia. Very high concentrations have a narcotic effect. The liquid is not highly irritating to the skin but can cause dermatitis.
화재위험
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
화학 반응
Reactivity with Water : No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by
ingestion and intraperitoneal routes. Mddly
toxic by skin contact. Human systemic
effects by inhalation: headache, nausea or
vomiting, eye and pulmonary changes. A
skin, mucous membrane, and severe eye
irritant. Can cause anemia and damage to
liver and hdneys. Narcotic in high
concentration. Flammable liquid when
exposed to heat or flame; can react with
oxidzing materials. Explosive in the form of
vapor when exposed to heat or flame. To
fight fire, use alcohol foam, foam, CO2, dry
chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See
also KETONES.
잠재적 노출
It is used as a solvent for pigments,
cellulose esters; oils and fats. It is used in hydraulic brake
fluids and in antifreeze formulations.
Carcinogenicity
Occupational exposure to 4-hydroxy-4-methyl-2-
pentanone is most likely to be by inhalation and skin contact.
It presents a low degree of hazard if good work practices are
observed. Appropriate protective clothing and eye protection
should be made available as prolonged exposure may defat the skin and cause dermatitis. The occurrence of eye, nose,
and throat irritation and a recognizable odor at low concentrations
should protect against overexposure to 4-hydroxy-4-
methyl-2-pentanone.
환경귀착
Biological. Using the BOD technique to measure biodegradation, the mean 5-d BOD value (mM
BOD/mM diacetone alcohol) and ThOD were 3.67 and 45.9%, respectively (Vaishnav et al.,
1987).
Photolytic. Grosjean (1997) reported a rate constant of 4.0 x 10
-12 cm
3/molecule?sec at 298 K for
the reaction of OH radicals in the atmosphere. Based on a OH concentration of 1.0 x 106
molecule/cm
3, the reported half-life of diacetone alcohol is 2.0 d (Grosjean, 1997).
운송 방법
UN1148 Diacetone alcohol, Hazard Class: 3;
Labels: 3-Flammable liquid.
Purification Methods
The pentanone loses water when heated. It can be dried with CaSO4, then fractionally distilled under reduced pressure. [Beilstein 1 IV 403.]
비 호환성
Vapor may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, perox-
ides, permanganates, perchlorates, chlorine, bromine, fluo-
rine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, and epoxides.
폐기물 처리
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinera-
tor equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be observed.
디아세톤알코올 준비 용품 및 원자재
원자재
준비 용품
산화 메시틸
PAINT
메틸이소부틸카비놀
2,4-다이메틸-3-사이클로헥신카복스알데하이드
2,2,4,4,6-pentamethyl-hexahydropyrimidine
오도라틴 (Odoratine)
2,8-Dimethylquinoline
6-Fluoroquinaldine
6-브로모-2-메틸퀴놀린
3,3,5,5-Tetramethyl-1,2,4-trithiolane
6-CHLORO-2-METHYLQUINOLINE