| Company Name: |
|
| Tel: |
|
| Email: |
bang.manoharlal@gmail.com |
| Products Intro: |
Cas:1538-09-6
ProductName:Benzathine penicillin
Purity: 98% | Package: 1 kg,5 kg, 10 kg,25kg and 1 MT
|
| Company Name: |
|
| Tel: |
|
| Email: |
jinesh@dodhiagroup.com |
| Products Intro: |
Cas:1538-09-6
ProductName:Benzathine penicillin
Purity: 99% | Package: 1 kg,5 kg, 10 kg,25kg
|
| Company Name: |
QUALITY CONTROL SOLUTIONS LTD.
|
| Tel: |
13670046396 |
| Email: |
sales@chem-strong.com |
| Products Intro: |
Cas:1538-09-6
ProductName:Benzathine penicillin
Purity: 95% HPLC | Package: 10MG;25MG;50MG;100MG
|
| Company Name: |
Wuhan Xiju Biotechnology Co., Ltd
|
| Tel: |
13001802035 |
| Email: |
2307019145@qq.com |
| Products Intro: |
Cas:1538-09-6
ProductName:Penicillin G benzathine
Purity: 99% | Package: 25kg/RMB 88
|
- Benzathine Penicillin
-

- $80.00 / 1KG
-
2024-03-26
- CAS:1538-09-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000KG
- Benzathine Penicillin
-

- $10.00 / 1KG
-
2021-08-31
- CAS:1538-09-6
- Min. Order: 1KG
- Purity: 99.99%
- Supply Ability: 20 tons/month
|
| Product Name: | Benzathine Penicillin | | Synonyms: | PENICILLIN-G BENZATHINE SALT;n,n'-dibenzylethylethylenediamine bis(benzylpenicillin);(2s,5r,6r)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid with n,n'-dibenzylethylenediamine (2:1);(2s,5r,6r)-3,3-dimethyl-7-oxo-6-[(phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;benzathine benzylpenicillin;BENZYLPENICILLIN BENZATHINE SALT;N,N-dibenzylethylenediammonium bis(6-benzylpenicillanate);beacillin | | CAS: | 1538-09-6 | | MF: | C48H56N6O8S2 | | MW: | 909.12 | | EINECS: | 216-260-5 | | Product Categories: | Organics;1538-09-6 | | Mol File: | 1538-09-6.mol | 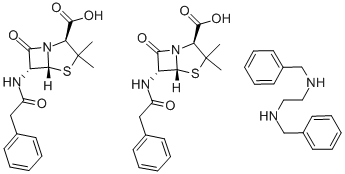 |
| | Benzathine Penicillin Chemical Properties |
| Hazard Codes | Xn | | Risk Statements | 22-42/43 | | Safety Statements | 36 | | WGK Germany | 3 | | RTECS | XH9425000 | | Toxicity | LD50 orl-mus: 2000 mg/kg NIIRDN 6,774,82 |
| | Benzathine Penicillin Usage And Synthesis |
| Description | Benzathine penicillin and procaine penicillin is an antibiotic that fights bacteria in your body. Benzathine penicillin and procaine penicillin is used to treat many different types of severe infections, including strep and staph infections, diphtheria, meningitis, gonorrhea, and syphilis. It is also used to prevent rheumatic fever. It is given by injection into a muscle. Belonging to a penicillin-class drug, its mechanism of action is inhibiting the cell wall synthesis of bacteria, further killing bacteria.
| | Chemical Properties | White or almost white powder. | | Originator | Bicillin,Wyeth,US,1951 | | Uses | The benzathine salt of penicillin G, an antibiotic used in the treatment of various bacterial infections, including Staphylococcus aureus. | | Uses | Benzathine benzylpenicillin is used as a Penicillin antibacterial. | | Manufacturing Process | Ethylenediamine (15 g, 0.25 mol) was added dropwise to 100 ml 98-100% formic acid in a two-necked 500 ml flask, fitted with an addition tube and reflux condenser with drying tube, cooled in an ice-bath. After complete addition of the base, 53 g of benzaldehyde (0.5 mol) was added in one lot. The ice-bath was removed and the flask was heated to the refluxing temperature. The initial rate of carbon dioxide evolution was too rapid to measure. After twenty minutes, the rate was circa 100 ml per minute and decreased rapidly to 8 ml per minute in one hour. Heating at reflux was continued for 35 hours.
Following the refluxing most of the excess formic acid was removed under reduced pressure. Hydrochloric acid (200 ml 6 N) was added to the viscous amber residue and heated under reflux, After 15 minutes, bumping necessitated cooling and filtering to remove crystalline dihydrochloride, which after washing with isopropanol was dried, MP circa 300°C. The mother liquors were refluxed one hour and cooled, obtaining an additional amount of product, MP circa 300°C. The filtrate was concentrated in vacuo to 100 ml, cooled and made alkaline with 40% NaOH. The supernatant oil was extracted with ether, dried, and fractionated from a stillpot packed with glass wool and heated in a sand-bath at 320°C. The first fraction at 106°C at 0.6-0.7 mm was Nbenzylethylenediamine (dipicrate, MP 222°C). The N,N'dibenzylethylenediamine was collected at 177°C to 206°C at 0.6-1.0 mm as a colorless liquid.
To a solution of 60 g of sodium penicillin G in 800 cc of distilled water cooled to 0°C to 4°C in an ice-bath, a solution of 35 g of N,N'dibenzylethylenediamine diacetate in 200 cc of distilled water is added dropwise with stirring. The thick slurry is filtered with suction, washed twice with 100 cc of cold water, dried by suction and spread out in a thin layer for completion of drying. The product weighed 80 g.
The air-dried powder has a broad melting point, sintering at 100°C, melting above 110°C to a cloudy liquid becoming clear at 135°C. | | Therapeutic Function | Antibacterial | | Clinical Use | Since penicillin G benzathine, N,N'-dibenzylethylenediaminedipenicillin G (Bicillin, Permapen), is the salt of a diamine,2 moles of penicillin are available from each molecule. It isvery insoluble in water, requiring about 3,000 mL to dissolve1 g. This property gives the compound great stabilityand prolonged duration of effect. At the pH of gastric juice,it is quite stable, and food intake does not interfere with itsabsorption. It is available in tablet form and in several parenteralpreparations. The activity of penicillin G benzathineis equivalent to 1,211 units/mg.Several other amines have been used to make penicillinsalts, and research is continuing on this subject. Otheramines that have been used include 2-chloroprocaine; L-Nmethyl-1,2-diphenyl-2-hydroxyethylamine (L-ephenamine);dibenzylamine; tripelennamine (Pyribenzamine); and N,N'-bis-(dehydroabietyl)ethylenediamine (hydrabamine). | | Safety Profile | Moderately toxic by ingestion andintraperitoneal routes. Experimental reproductive effects.When heated to decomposition it emits very toxic fumesof NOx and SOx. See other penicillin entries. |
| | Benzathine Penicillin Preparation Products And Raw materials |
|