- α-Cyclodextrine
-

- $50.00 / 1kg
-
2024-04-19
- CAS:10016-20-3
- Min. Order: 1kg
- Purity: 99.10%
- Supply Ability: 5000kg
- α-Cyclodextrine
-

- $3.00 / 1kg
-
2024-04-12
- CAS:10016-20-3
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 10 tons
- α-Cyclodextrine
-

- $5.00 / 1KG
-
2024-03-26
- CAS:10016-20-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available
|
| | α-Cyclodextrine Basic information |
| Product Name: | α-Cyclodextrine | | Synonyms: | CYCLOMALTOHEXAOSE;CYCLOMALTOHEXOSE;2,4,7,9,12,14,17,19,22,24,27,29-Dodecaoxaheptacyclo(26.2.2.23,6.28,11.213,16.218,21.223,26)dotetracontane-31,32,33,34,35,36,37,38,39,40,41,42-dodecol, 5,10,15,20,25,30-hexakis(hydroxymethyl)-;alpha-Cycloamylose;alpha-Dextrin;alpha-Schardinger dextrin;alpha-schardingerdextrin;Cyclohexaamylose carbonate | | CAS: | 10016-20-3 | | MF: | C36H60O30 | | MW: | 972.84 | | EINECS: | 233-007-4 | | Product Categories: | Industrial/Fine Chemicals;Biochemistry;Cyclodextrins;Functional Materials;Macrocycles for Host-Guest Chemistry;Oligosaccharides;Sugars;Dextrins、Sugar & Carbohydrates;10016-20-3 | | Mol File: | 10016-20-3.mol | 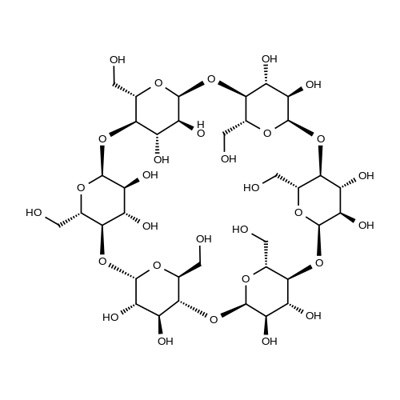 |
| | α-Cyclodextrine Chemical Properties |
| Melting point | >278 °C (dec.) (lit.) | | alpha | [α]D25 +146~+151° (c=1, H2O) (After Drying) | | Boiling point | 784.04°C (rough estimate) | | density | 1.2580 (rough estimate) | | refractive index | 1.7500 (estimate) | | storage temp. | 2-8°C | | solubility | H2O: 50 mg/mL | | form | powder | | pka | 11.77±0.70(Predicted) | | color | White | | PH | 5.0-8.0 (1% in solution) | | Odor | at 100.00?%. odorless | | optical activity | [α]20/D +136±3°, c = 10% in H2O | | Water Solubility | Soluble in water at 1%(w/v) | | Merck | 14,2718 | | BRN | 4227442 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | HFHDHCJBZVLPGP-RWMJIURBSA-N | | LogP | -7.77 | | CAS DataBase Reference | 10016-20-3 | | NIST Chemistry Reference | «alpha»-Cyclodextrin(10016-20-3) | | EPA Substance Registry System | .alpha.-Cyclodextrin (10016-20-3) |
| Hazard Codes | Xi | | Risk Statements | 36-36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | RTECS | GU2292000 | | TSCA | Yes | | HS Code | 29400090 |
| | α-Cyclodextrine Usage And Synthesis |
| Description |
α-Cyclodextrine (α-CD) is a cyclic oligosaccharide derived from corn (Trade name: Mirafit fbcx, ArtJen Complexus, Windsor, Ontario, Canada). It has been shown to form a stable complex with dietary fat. This complex is resistant to normal lipolytic hydrolysis by lipases and thereby reduces the absorption and bioavailability of dietary fat[1].
| | Chemical Properties | White crystalline powder | | Chemical Properties | Cyclodextrins occur as white, practically odorless, fine crystalline
powders, having a slightly sweet taste. Some cyclodextrin
derivatives occur as amorphous powders. | | Uses | A naturally occuring clathrate. | | Uses | also available in pharma grade | | Uses | Useful for selective precipitation of enantiomeric, positional or structural isomersα-Cyclodextrin is used as a fiber ingredient, an odor or flavor masking agent. It is also useful for emulsification applications. It is also used as whipping fiber and emulsifying fiber. It finds application in medical, healthcare and food and beverage applications. It is also used to lower blood low-density lipoprotein cholesterol levels and lower blood triglyceride levels. It plays an essential role in fat free or fat containing dessert compositions and also employed for the reduction or the replacement of egg white in confectionary and bakery applications. Further, it acts as a supramolecular carrier, complexing agent and controlled drug release. In addition to this, it is used to increase the insulin and leptin sensitivity. | | Definition | ChEBI: Alpha-cyclodextrin is a cycloamylose composed of six alpha-(1->4) linked D-glucopyranose units. | | Production Methods | Cyclodextrins are manufactured by the enzymatic degradation of
starch using specialized bacteria. For example, β-cyclodextrin is
produced by the action of the enzyme cyclodextrin glucosyltransferase
upon starch or a starch hydrolysate. An organic solvent is
used to direct the reaction that produces β-cyclodextrin, and to
prevent the growth of microorganisms during the enzymatic
reaction. The insoluble complex of β-cyclodextrin and organic
solvent is separated from the noncyclic starch, and the organic
solvent is removed in vacuo so that less than 1 ppm of solvent
remains in the β-cyclodextrin. The β-cyclodextrin is then carbon
treated and crystallized from water, dried, and collected. | | General Description | Hexagonal plates or blade-shaped needles. | | Reactivity Profile | Cyclohexapentylose has hydrophobic cavities. Cyclohexapentylose forms inclusion compounds with organic substances, salts, and halogens in the solid state or in aqueous solutions. Cyclohexapentylose is incompatible with strong oxidizing agents. | | Fire Hazard | Flash point data for Cyclohexapentylose are not available; however, Cyclohexapentylose is probably combustible. | | Flammability and Explosibility | Non flammable | | Pharmaceutical Applications | Cyclodextrins (α-Cyclodextrine) are ‘bucketlike’ or ‘conelike’ toroid molecules, with a rigid structure and a central cavity, the size of which varies according to the cyclodextrin type. The internal surface of the cavity is hydrophobic and the outside of the torus is hydrophilic; this is due to the arrangement of hydroxyl groups within the molecule. This arrangement permits the cyclodextrin to accommodate a guest molecule within the cavity, forming an inclusion complex.Cyclodextrins may be used to form inclusion complexes with a variety of drug molecules, resulting primarily in improvements to dissolution and bioavailability owing to enhanced solubility and improved chemical and physical stability.
Cyclodextrin inclusion complexes have also been used to mask the unpleasant taste of active materials and to convert a liquid substance into a solid material.
a-Cyclodextrin is used mainly in parenteral formulations.
However, as it has the smallest cavity of the cyclodextrins it can
form inclusion complexes with only relatively few, small-sized
molecules. In contrast, g-cyclodextrin has the largest cavity and can
be used to form inclusion complexes with large molecules; it has low
toxicity and enhanced water solubility.
In parenteral formulations, cyclodextrins have been used to produce stable and soluble preparations of drugs that would otherwise have been formulated using a nonaqueous solvent.
In eye drop formulations, cyclodextrins form water-soluble complexes with lipophilic drugs such as corticosteroids. They have been shown to increase the water solubility of the drug; to enhance drug absorption into the eye; to improve aqueous stability; and to reduce local irritation.
Cyclodextrins have also been used in the formulation of solutions,suppositories, and cosmetics.
| | Biochem/physiol Actions | α-Cyclodextrin is found to form a firm complex with dietary fats. This way it decreases the bioavailability and absorption of fats. It is known to regulate triglyceride and leptin levels in serum. In rat models, α-Cyclodextrin is shown to induce insulin sensitivity and fecal fat excretion. Thus, α-cyclodextrin is considered to be effective for treating obesity and metabolic syndromes. | | Safety | Cyclodextrins are starch derivatives and are mainly used in oral and
parenteral pharmaceutical formulations. They are also used in
topical and ophthalmic formulations.
Cyclodextrins are also used in cosmetics and food products, and
are generally regarded as essentially nontoxic and nonirritant
materials. However, when administered parenterally, β-cyclodextrin
is not metabolized but accumulates in the kidneys as insoluble
cholesterol complexes, resulting in severe nephrotoxicity.
Cyclodextrin administered orally is metabolized by microflora in
the colon, forming the metabolites maltodextrin, maltose, and
glucose; these are themselves further metabolized before being
finally excreted as carbon dioxide and water. Although a study
published in 1957 suggested that orally administered cyclodextrins
were highly toxic, more recent animal toxicity studies in rats and
dogs have shown this not to be the case, and cyclodextrins are now
approved for use in food products and orally administered
pharmaceuticals in a number of countries.
Cyclodextrins are not irritant to the skin and eyes, or upon
inhalation. There is also no evidence to suggest that cyclodextrins
are mutagenic or teratogenic.
α-Cyclodextrin
LD50 (rat, IP): 1.0 g/kg(15)
LD50 (rat, IV): 0.79 g/kg | | storage | Cyclodextrins should be stored in a tightly sealed container, in a
cool, dry place.Cyclodextrins are stable in the solid state if
protected from high humidity. | | Purification Methods | Recrystallise α-cyclodextrin from 60% aqueous EtOH, then twice from water, and dry it for 12hours in a vacuum at 80o. It is also purified by precipitation from water with 1,1,2-trichloroethylene. The precipitate is collected, washed and resuspended in water. This is boiled to steam distil the trichloroethylene. The solution is then freeze-dried to recover the cyclodextrin. [Armstrong et al. J Am Chem Soc 108 1418 1986]. [Beilstein 19/12 V 789.] | | Regulatory Status | Included in the FDA Inactive Ingredients Database: α-cyclodextrin
(injection preparations); β-cyclodextrin (oral tablets, topical gels);
γ-cyclodextrin (IV injections).
Included in the Canadian List of Acceptable Non-medicinal
Ingredients (stabilizing agent; solubilizing agent ); and in oral and
rectal pharmaceutical formulations licensed in Europe, Japan, and
the USA. | | References | [1] Kevin B. Comerford. “The Beneficial Effects α-Cyclodextrin on Blood Lipids and Weight Loss in Healthy Humans.” Obesity 19 6 (2012): 1200–1204. |
| | α-Cyclodextrine Preparation Products And Raw materials |
|