- Diflorasone
-
- $1020.00 / 100gram
-
2023-11-27
- CAS:2557-49-5
- Min. Order: 100gram
- Purity: 99% HPLC
- Supply Ability: 20 tons
- Diflorasone
-

- $10.00 / 1kg
-
2023-08-29
- CAS:2557-49-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000tons
- Diflorasone
-

- $0.00 / 25KG
-
2023-05-26
- CAS:2557-49-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TON
|
| | Diflorasone Basic information |
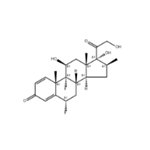
| Product Name: | Diflorasone | | Synonyms: | (6s,8s,9s,10s,11s,13s,14s,16s,17r)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one;DIFLORASONE;(6S,8S,9R,10S,11S,13S,14S,16S,17R)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one;(6S,8S,9R,10S,11S,13S,14S,16S,17R)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyethanoyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one;(6S,8S,9R,10S,11S,13S,14S,16S,17R)-6,9-difluoro-17-glycoloyl-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one;(6α,11β,16β)-6,9-Difluoro-11,17,21-trihydroxy-16-Methyl-pregna-1,4-diene-3,20-dione;Difloasone;Diflorasone [INN:BAN] | | CAS: | 2557-49-5 | | MF: | C22H28F2O5 | | MW: | 410.46 | | EINECS: | 219-875-7 | | Product Categories: | Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;Steroids | | Mol File: | 2557-49-5.mol | 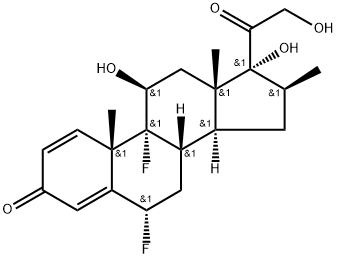 |
| | Diflorasone Chemical Properties |
| Melting point | 228-239°C | | Boiling point | 569.8±50.0 °C(Predicted) | | density | 1.36±0.1 g/cm3(Predicted) | | storage temp. | Refrigerator | | solubility | Methanol (Slightly) | | pka | 11.98±0.70(Predicted) | | form | Solid | | color | Off-White to Pale Yellow | | CAS DataBase Reference | 2557-49-5 |
| | Diflorasone Usage And Synthesis |
| Chemical Properties | Off-White Solid | | Originator | Florone,Upjohn,US,1978 | | Uses | An anti-inflammatory and anti-itching corticosteroid usually present in topical creams. | | Definition | ChEBI: The 16beta-analogue of flumethasone. It is used as the 17,21-diacetate as a topical anti-inflammatory and antipruritic in the treatment of various skin disorders. | | Manufacturing Process | 6α-Fluoro-9β-epoxy-17α,21-dihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione-21-acetate:To a solution of 6.78 g of 6α-fluoro-9α-bromo-11β,17α,21-
trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-acetate in 175 ml of
acetone was added 6.78 g of potassium acetate and the resulting suspension
was heated under reflux for a period of 17 hours. The mixture was then
concentrated to approximately 60 ml volume at reduced pressure on the
steam bath, diluted with water and extracted with methylene chloride. The
methylene chloride extracts were combined, washed with water, dried over
anhydrous sodium sulfate and evaporated. The residue was redissolved in
methylene chloride and chromatographed over 500 g of Florisil anhydrous
magnesium silicate. The column was eluted with 1 liter portions of hexanes
(Skellysolve B) containing increasing proportions of acetone. There was so
eluted 6α-fluoro-9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-
pregnadiene-3,20-dione-21-acetate which was freed of solvent by evaporation
of the eluates.
6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione-2-1-acetate: To approximately 1.3 g of hydrogen fluoride contained in a
polyethylene bottle and maintained at -60C was added 2.3 ml of
tetrahydrofuran and then a solution of 500 mg (0,0012 mol) of 6α-fluoro9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-pregnadiene-3,20-dione-21-
acetate in two ml of methylene chloride. The steroid solution was rinsed in
with an additional 1 ml of methylene chloride. The light red colored solution
was then kept at approximately -30°C for 1 hour and at -10°C for 2 hours. At
the end of this period it was mixed cautiously with an excess of cold sodium
bicarbonate solution and the organic material extracted with the aid of
additional methylene chloride.
The combined extracts were washed with water, dried over anhydrous sodium
sulfate and concentrated to approximately 35 ml. The solution was
chromatographed over 130 g of Florisil anhydrous magnesium silicate. The
column was developed with 260 ml portions of hexanes (Skellysolve B)
containing increasing proportions of acetone. There was thus eluted 6α,9αdifluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-
acetate which was freed of solvent by evaporation of the eluate fractions.
6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione: 3.25 g of 6α,9α-difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-
pregnadiene-3,20-dione-21-acetate was dissolved in 325 ml of methanol,
previously purged of air-oxygen by passing nitrogen through it for 10 minutes
and thereto was added a solution of 1.63 g of potassium bicarbonate in 30 ml
of water, similarly purged of oxygen. The mixture was allowed to stand at
room temperature for a period of 5 hours in a nitrogen atmosphere,
thereupon neutralized with 2.14 ml of acetic acid in 40 ml of water. The
mixture was concentrated to approximately one-third volume at reduced
pressure on a 60°C water bath. Thereupon 250 ml of water was added and
the mixture chilled. The crystalline product was collected on a filter, washed
with water and dried to give 6α,9α-difluoro-11β,17α,21-trihydroxy-16αmethyl-1,4-pregnadiene-3,20-dione.
The diflorasone is reacted with orthoacetic acid trimethyl ester in the presence
of toluenesulfonic acid to give diflorasone diacetate. | | Brand name | Florone (Pharmacia & Upjohn); Psorcon (Pharmacia & Upjohn); Psorcon (Sanofi Aventis). | | Therapeutic Function | Antiinflammatory |
| | Diflorasone Preparation Products And Raw materials |
|