| Company Name: |
Leancare Ltd.
|
| Tel: |
+33 962096793 |
| Email: |
enquiry@leancare.co.uk |
| Products Intro: |
|
| Company Name: |
2A PharmaChem USA
|
| Tel: |
(630) 737-0988 |
| Email: |
sales@2apharmachem.com |
| Products Intro: |
|
|
| | ioglycamic acid Basic information |
| Product Name: | ioglycamic acid | | Synonyms: | BE-419;3-[[2-[2-(3-carboxy-2,4,6-triiodo-anilino)-2-keto-ethoxy]acetyl]amino]-2,4,6-triiodo-benzoic acid;3-[[2-[2-(3-carboxy-2,4,6-triiodoanilino)-2-oxoethoxy]acetyl]amino]-2,4,6-triiodobenzoic acid;3-[2-[2-[(3-carboxy-2,4,6-triiodo-phenyl)amino]-2-oxo-ethoxy]ethanoylamino]-2,4,6-triiodo-benzoic acid;Benzoic acid,3,3'-[oxybis[(1-oxo-2,1-ethanediyl)iMino]]bis[2,4,6-triiodo-;Ioglycamic;3,3'-[Oxybis[(1-oxo-2,1-ethanediyl)imino]]bis[2,4,6-triiodobenzoic acid];Ioglycamide | | CAS: | 2618-25-9 | | MF: | C18H10I6N2O7 | | MW: | 1127.71 | | EINECS: | 220-048-8 | | Product Categories: | | | Mol File: | 2618-25-9.mol | 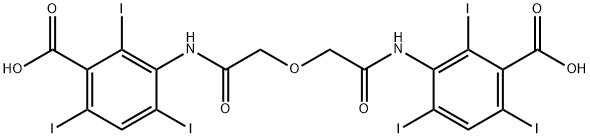 |
| | ioglycamic acid Chemical Properties |
| Melting point | with baking at 222° | | Boiling point | 882.36°C (rough estimate) | | Water Solubility | 0.2g/L(temperature not stated) |
| | ioglycamic acid Usage And Synthesis |
| Originator | Biligram,Schering,W. Germany,1971 | | Uses | Diagnostic aid (radiopaque medium, cholecystographic). | | Definition | ChEBI: Ioglycamic acid is an amidobenzoic acid. | | Manufacturing Process | 910 g of dry 2,4,6-triiodo amino benzoic acid are dissolved with stirring in
4,800 cc of dry, boiling chlorobenzene. A solution of 151.7 g diglycolic acid
dichloride in 100 cc of dry chlorobenzene is slowly added to this solution and
the mixture is further heated for 4 to 5 hours under reflux until development
of hydrogen chloride has ceased. The resulting precipitate is filtered from the
warm solution with suction and washed with chlorobenzene and then with
ether. The microcrystalline, almost colorless crude product, 942 g, consists of
the a-modification of diglycolic acid di-(3-carboxy-2,4,6-triiodo anilide).
The crude product is suspended, while stirring, in 2.5 liters of pure methanol
and a solution of 73 g of pure sodium hydroxide in the same weight of water,
diluted with 675 cc methanol, is slowly added to this suspension until the acid
is dissolved and the pH of this solution reaches 9.0. The solution is allowed to
stand at this pH for 15 minutes. The pH is then brought to 4.0 by addition of
10% acetic acid and 17 g of charcoal are stirred in. After 15 minutes the coal
is filtered off and the clear filtrate is slowly added to a stirred solution of 415
cc of pure, concentrated hydrochloric acid in 4.15 liters of 50% methanol.
After ? hour of stirring and decanting after 1 hour, the precipitate is easily
filtered off with suction, washed with little methanol and thoroughly with
water, until the thixotropic residue is free of hydrochloric acid. In order to
obtain a product of highest purity, this treatment is repeated two times. The
resulting pure product, after drying in vacuo at 50°C still containing one
molecule of methanol per two molecules of the acid (plus 4 molecules of
water), must be suspended in boiling water and steamed out. The hot
suspension is filtered with suction, the white microcrystalline residue is dried
in vacuo at 50°C to give 860 g (83.5% of the theoretical yield) of the pure
dihydrate of the diglycolic acid di-(3-carboxy-2,4,6-triiodo anilide), β-
modification. | | Therapeutic Function | Diagnostic aid (radiopaque medium) |
| | ioglycamic acid Preparation Products And Raw materials |
|