| Company Name: |
3B Pharmachem (Wuhan) International Co.,Ltd.
|
| Tel: |
821-50328103-801 18930552037 |
| Email: |
3bsc@sina.com |
| Products Intro: |
Product Name:MEDRYSONE
CAS:2668-66-8
Purity:99% HPLC Package:1Mg ; 5Mg;10Mg ;100Mg;250Mg ;500Mg ;1g;2.5g ;5g ;10g
|
| Company Name: |
Sinopharm Chemical Reagent Co,Ltd.
|
| Tel: |
86-21-63210123 |
| Email: |
sj_scrc@sinopharm.com |
| Products Intro: |
Product Name:Medrysone (500 mg)
CAS:2668-66-8
Package:500 mg
|
|
| | MEDRYSONE Basic information |
| Product Name: | MEDRYSONE | | Synonyms: | 11BETA-HYDROXY-6ALPHA-METHYL-4-PREGNENE-3,20-DIONE;4-PREGNEN-6-ALPHA-METHYL-11-BETA-OL-3,20-DIONE;6ALPHA-METHYL-11BETA-HYDROXYPROGESTERONE;MEDRYSONE;11-beta-hydroxy-6-alpha-methyl-pregn-4-ene-20-dione;11-beta-hydroxy-6-alpha-methylpregn-4-ene-3,20-dione;hmsliquifilm;nsc-63278 | | CAS: | 2668-66-8 | | MF: | C22H32O3 | | MW: | 344.5 | | EINECS: | 220-208-7 | | Product Categories: | Organics;Steroids;HMS | | Mol File: | 2668-66-8.mol | 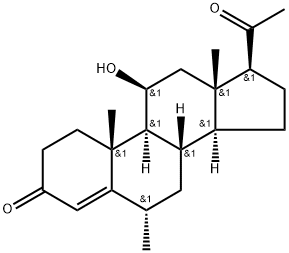 |
| | MEDRYSONE Chemical Properties |
| Melting point | 155-158° | | alpha | D +189° (in chloroform) | | Boiling point | 492.3±45.0 °C(Predicted) | | density | 1.13±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | Acetone (Slightly), Chloroform (Slightly), Methanol (Slightly, Sonicated) | | form | Solid | | pka | 14.53±0.70(Predicted) | | color | White to Off-White |
| WGK Germany | 3 | | RTECS | TU5250000 |
| | MEDRYSONE Usage And Synthesis |
| Description | 6α-methyl-11β-hydroxy Progesterone is an anti-inflammatory steroid. It is a hydroxylated progesterone that lacks progestational and androgenic activity. It inhibits phytohemagglutinin-activated T cell proliferation in vitro with an IC50 value of 2.9 μg/ml. 6α-methyl-11β-hydroxy Progesterone also inhibits TNF-α production in LPS-stimulated THP-1 cells (IC50 = 30.54 μg/ml). Topical formulations containing 6α-methyl-11β-hydroxyprogesterone have been used to treat inflammatory ocular diseases. | | Originator | HMS,Allergan,US,1970 | | Uses | glucocorticoid | | Uses | Medrysone is a corticosteroid that is seen to increase intraocular pressure. | | Definition | ChEBI: Medrysone is a corticosteroid hormone. | | Manufacturing Process | Preparation of 11-Keto-6β-Methylprogesterone 3,20-bis-(Ethylene Ketal): A
mixture of 5 g of 11-keto-6β-methylprogesterone [Spero et al, A Am. Chem.
Soc., 78, 6213 (1956)], 503 ml of benzene, 26 ml of ethylene glycol, and
0.152 g of p-toluenesulfonic acid monohydrate was stirred and heated under
reflux for 22 hours while water was removed by means of a water trap. The
reaction mixture was then cooled to 30°C, 0.4 ml of pyridine was added, and
stirring was continued for 10 minutes.
The reaction mixture was then shaken with 110 ml of water and the organic
and aqueous layers separated. The organic layer was dried over sodium
sulfate and evaporated under diminished pressure giving a residue. The thus
obtained residue was recrystallized from methanol giving 2.68 g of 11-keto6β-methyl progesterone 3,20-bis-(ethylene ketal) having a MP of 168° to
175°C.
Preparation of 11β-Hydroxy-6α-Methylprogesterone: A mixture of 2.68 g of 11-keto-6β-methylprogesterone 3,20-bis-(ethylene ketal), 161 ml of
tetrahydrofuran (previously distilled from lithium aluminum hydride), 1.34 g of
lithium aluminum hydride and 14.5 ml of absolute ether was stirred and
refluxed under nitrogen for 1.5 hours, then 27 ml of water was added
cautiously, to decompose excess hydride. The resulting mixture was filtered
and the filter cake was washed with 135 ml of ether. The combined filtrate
and wash was shaken with 135 ml of water and separated. The aqueous layer
was washed with four 55-ml portions of ether, then the organic layer and the
washes were combined, washed once with water, and evaporated to dryness
under diminished pressure leaving a tan residue.
The thus-obtained residue was dissolved in a mixture of 268 ml of methanol
and 26.8 mi of 3 N aqueous sulfuric acid and heated under reflux for 40
minutes, with a color change from yellow to green. The reaction mixture was
then cooled, neutralized by addition of 127 ml of 5% sodium bicarbonate
solution, and concentrated under reduced pressure until almost all the
methanol was removed. The resulting solid was removed by filtration, washed
with water, dried, and twice crystallized from ethyl acetate to give 1.1 g of
11β-hydroxy-6α-methylprogesterone having a MP of 155° to 158°C, according
to US Patent 2,864,837. | | Therapeutic Function | Glucocorticoid | | references | [1] the dictionary of drugs: chemical data: chemical data, structures and bibliographies[m]. springer, 2014.
[2] spaeth g l. hydroxymethylprogesterone: an anti-inflammatory steroid without apparent effect on intraocular pressure[j]. archives of ophthalmology, 1966, 75(6): 783-787. |
| | MEDRYSONE Preparation Products And Raw materials |
|