- Lily aldehyde
-

- $120.00 / 1kg
-
2024-04-25
- CAS:80-54-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Lily aldehyde
-

- $80.00 / 1kg
-
2024-04-25
- CAS:80-54-6
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 5000
- Lily aldehyde
-

- $0.00 / 1kg
-
2023-06-26
- CAS:80-54-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500000kg
|
| Product Name: | Lily aldehyde | | Synonyms: | HYDROCINNAMALDEHYDE,PARA-TERT-BUTYL-ALPHA-METHYL-;PARA-TERT-BUTYL-ALPHA-METHYLHYDROCINNAMICALDEHYDE;2-(4-tert-Butylbenzyl)propionaldehyd;2-(4-tert-Butylbenzyl)propionaldehyde
3-(4-tert-Butylphenyl)isobutyraldehyde
3-(4-tert-Butylphenyl)-2-methylpropionaldehyde
4-tert-Butyl-α-methylhydrocinnamaldehyde
4-tert-Butyl-α-methyl-benzenepropanal;P-TERT-BUTYL-A-METHYLHYDROCINN AMALDEHYDE;4-TERT-BUTYL-ALPHA-METHYL-BENZENEPROPANAL;4-TERT-BUTYL-ALPHA-METHYLHYDROCINNAMALDEHYDE;3-(4-TERT-BUTYLPHENYL)ISOBUTYRALDEHYDE | | CAS: | 80-54-6 | | MF: | C14H20O | | MW: | 204.31 | | EINECS: | 201-289-8 | | Product Categories: | Organics | | Mol File: | 80-54-6.mol | 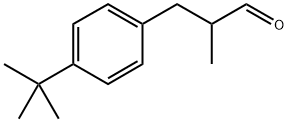 |
| | Lily aldehyde Chemical Properties |
| Melting point | 106-109 °C | | Boiling point | 150°C 10mm | | density | 0.946 g/mL at 20 °C(lit.) | | vapor pressure | 0.25Pa at 20℃ | | refractive index | n20/D 1.505 | | Fp | 100°C | | storage temp. | 2-8°C | | solubility | Chloroform (Sparingly), Ethyl Acetate (Slightly) | | color | Colorless to Light yellow | | Odor | at 100.00 %. floral muguet watery green powdery cumin | | Odor Type | floral | | Water Solubility | 33mg/L at 20℃ | | BRN | 880140 | | InChIKey | SDQFDHOLCGWZPU-UHFFFAOYSA-N | | LogP | 4.2 at 24℃ | | CAS DataBase Reference | 80-54-6(CAS DataBase Reference) | | NIST Chemistry Reference | Lilial(80-54-6) | | EPA Substance Registry System | Benzenepropanal, 4-(1,1-dimethylethyl)-.alpha.-methyl- (80-54-6) |
| | Lily aldehyde Usage And Synthesis |
| Description | Lily aldehyde (also known as lysmeral or Lilial) is a chemical compound commonly used as a perfume in cosmetic preparations and laundry powders1-3. It is fresh light green floral lily muguet lindenblossom aldehyde. Lilial is used in a wide variety of compositions where it confers a particularly recommended for floral notes such as muguet, linden-blossom and cyclamen2.
| | Chemical Properties | Lilial is a colorless to pale yellow liquid, as yet has not been found in nature. It has a higher stability than the homologous cyclamenaldehyde and therefore is used as scent in soaps.

Lilial is a widely used fragrance compound found naturally in the essential oil of chamomile and is used synthetically in a variety of beauty products, including perfumes, shampoos, deodorants, tanning lotions and hairstyling products, primarily for its Lily of the Valley aroma.
| | Chemical Properties | Lily aldehyde is a homolog of
cyclamenaldehyde. The racemic compound is a colorless to slightly yellow liquid
with a mild-floral odor, reminiscent of cyclamen and lily of the valley.
The aldehyde is prepared by the same routes as cyclamenaldehyde.
Other routes start from ??-methylcinnamaldehyde. ??-Methylcinnamaldehyde
(from benzaldehyde and propionaldehyde) is hydrogenated to ??-methyldihydrocinnamic
alcohol.The alcohol is alkylated with tert-butyl chloride or isobutene to
4-tert-butyl-??-methyldihydrocinnamic alcohol, which is subsequently dehydrogenated to the desired aldehyde.
4-tert-Butyl-??-methyldihydrocinnamaldehyde is more stable than cyclamenaldehyde
and is a popular component of flower compositions, particularly lily of the
valley and linden types, because of its mild, pleasant, blossomfragrance. It is used
in a wide range of perfume types, but especially in soap and detergent perfumes. | | Uses | In addition to its applications in the perfume and aroma industry, lilial is used mainly for the synthesis of substituted 3-(4-tert-butylphenyl)-2-methylpropylamines, a new class of substances with fungicidal properties. These compounds are effective against mildew in barley and wheat. | | Uses | 2-(4-tert-Butylbenzyl)propionaldehyde may be used as a reference standard for the determination of 2-(4-tert-butylbenzyl)propionaldehyde in:
- Human urine samples by ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) equipped with electrospray ionization (ESI) source and multiple reaction monitoring (MRM) mode of detection.
- Deodorants and air fresheners by sonication extraction coupled with gas chromatography-mass spectrometry (GC-MS).
- Scented consumer products by GC-MS as well as GC in combination with olfactometry.
| | Production Methods | Lily aldehyde is produced industrially almost solely by aldol condensation of 4-tert-butylbenzaldehyde and propionaldehyde to give 4-tert-butyl-α-methylcinnamaldehyde, which can be hydrogenated selectively on noble metal catalysts such as Pd, Rh, Pd – Pr2O3 on Al2O3, or on modified cobalt catalysts. The aldol condensation and the hydrogenation can be carried out in one step in the presence of a hydrogenation catalyst.
The Friedel – Crafts reaction of 4-tertbutylbenzene with methacrolein or methacrolein diacetate proceeds in an analogous manner to the preparation of cyclamenaldehyde.
Further possibilities are the Rh-catalyzed hydroformylation of 1-(4-tert-butylphenyl)-1- methoxypropene and subsequent partial hydrogenation, the palladium salt catalyzed reaction of 4-tert-butylphenylhalide with methallylalcohol, and the dehydrogenation of 3-(4-tert-butylphenyl)-2-methylpropanol on silver catalysts. | | General Description | 2-(4-tert-Butylbenzyl)propionaldehyde is categorized under the synthetic fragrance class of compounds widely utilized in consumer products such as perfumes, after shave lotions, cosmetics, etc. | | Flammability and Explosibility | Non flammable | | Trade name | Lilestralis Pure (Innospec), Lysmeral® Extra (BASF). | | Contact allergens | Lilial? is a synthetic compound listed as a fragrance
allergen. Its presence is indicated on cosmetics within
the EU. |
| | Lily aldehyde Preparation Products And Raw materials |
|