DESIPRAMINE manufacturers
- DESIPRAMINE USP/EP/BP
-
- $1.10 / 1g
-
2021-07-27
- CAS:50-47-5
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
|
| | DESIPRAMINE Basic information |
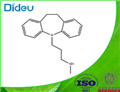
| Product Name: | DESIPRAMINE | | Synonyms: | TIMTEC-BB SBB002569;N-[3-(10,11-DIHYDRO-5H-DIBENZO[B,F]AZEPIN-5-YL)PROPYL]-N-METHYLAMINE HYDROCHLORIDE;DESIPRAMINE;AURORA KA-7618;10,11-Dihydro-5-(3-methylaminopropyl)-5H-dibenz(b,f)azepine;3-(10,11-Dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-methyl-1-propanamine;5H-Dibenz[b,f]azepine, 10,11-dihydro-5-[3-(methylamino)propyl]-;5H-Dibenz[b,f]azepine-5-propanamine, 10,11-dihydro-N-methyl- | | CAS: | 50-47-5 | | MF: | C18H22N2 | | MW: | 266.38 | | EINECS: | 200-040-0 | | Product Categories: | | | Mol File: | 50-47-5.mol |  |
| | DESIPRAMINE Chemical Properties |
| Melting point | 212°C | | Boiling point | bp0.02 172-174° | | density | 0.9934 (rough estimate) | | refractive index | 1.5200 (estimate) | | pka | pKa 10.21(-)(H2O t=20) (Uncertain) | | form | powder | | color | white | | EPA Substance Registry System | Desipramine (50-47-5) |
| | DESIPRAMINE Usage And Synthesis |
| Originator | Pertofran,Geigy,UK,1963 | | Uses | Desipramine is used for depression of various etiology and in particular for endogenous
depression. | | Uses | Antidepressant. | | Definition | ChEBI: A dibenzoazepine consisting of 10,11-dihydro-5H-dibenzo[b,f]azepine substituted on nitrogen with a 3-(methylamino)propyl group. | | Manufacturing Process | Oxidative coupling of o-nitrotoluene gives 4,4'-dinitrodibenzyl which is reduced
with hydrogen to the diamine. The diamine is pyrolyzed to give
dihydrobenzazepine. This is reacted with N-(3-chloropropyl)-Nmethylbenzamine to give N-benzyldesipramine. This is debenzylated by
reductive cleavage and then reacted with HCl. | | Brand name | Norpramin (Sanofi Aventis); Pertofrane (Sanofi Aventis). | | Therapeutic Function | Psychostimulant | | Mechanism of action | Its antidepressant effect results from increases in the level of NE in CNS synapses, and long-term
administration causes a downregulation of α1-adrenoceptors and desensitization of presynaptic α2-receptors, equilibrating
the noradrenergic system and, thus, correcting the dysregulated output of depressed patients. The SSRIs do
not produce this effect. Desipramine also downregulates the NET, but not the 5-SERT . Substantial loss of NE
transporter–binding sites takes 15 days to occur and is accompanied by a marked reduction of NET function
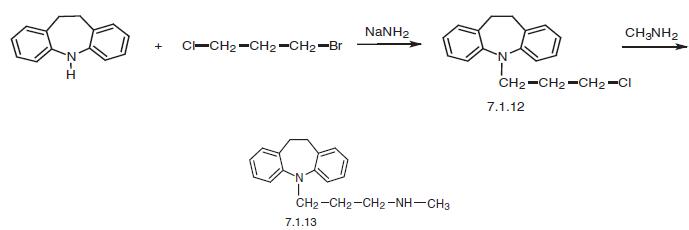
in vivo. Desipramine has weak effects on 5-HT reuptake. | | Synthesis | Desipramine, 10,11-dihydro-5-[3-(methylamino)propyl]-5H-dibenz[b,f] azepine (7.1.13), differs from imipramine in that it contains only one methyl group on the nitrogen atom of the propylamine side chain. The suggested methods of desipramine syn�thesis are very simple, and the difference lies only in the manner in which the secondary methylamine group is introduced into the structure of the drug.
The first way of synthesis is by the alkylation of 10,11-dihydro-5H-dibenz[b,f]azepine using 1-bromo-3-chloropropane in the presence of sodium amide into a chloro derivative (7.1.12) and the subsequent reaction of this with methylamine, giving desipramine (7.1.13) [18–20].
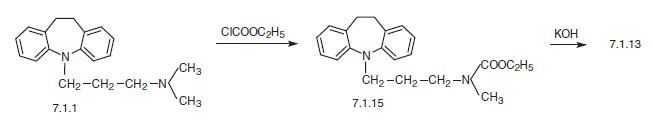
Finally, a second way of synthesis is from imipramine (7.1.1), which undergoes demethylation by successive reaction with ethyl chloroformate, giving 5-[3-(N-carbethoxy-N-methyl)amino�propyl]-10,11-dihydro-5H-dibenz[b,f]azepine (7.1.15), the alkaline hydrolysis of which leads to desipramine (7.1.13) [23,24].
 | | Metabolism | Desipramine is a dihydrodibenzazepine secondary amine TCA that also is the active metabolite of imipramine. Desipramine appears to have a bioavailability comparable to the other secondary TCAs. Desipramine is distributed into milk in concentrations similar to those present at steady state in
maternal plasma. This drug is metabolized primarily by CYP2D6 to its 2-hydroxy metabolite and by CYP1A2
and CYP2C19 to its N-demethylated (primary amine) metabolite .
Desipramine exhibits a greater potency and selectivity for the NET than the other secondary TCAs do. |
| | DESIPRAMINE Preparation Products And Raw materials |
|