|
|
| | CHLOZOLINATE Basic information |
| Product Name: | CHLOZOLINATE | | Synonyms: | CHLOZOLINATE;SERINAL;ethyl (RS)-3-(3,5-dichlorophenyl)-5-methyl-2,4-dioxooxazolidine-5-carboxylate;chlozolinate (bsi,iso);ethyl 3-(3,5-dichlorophenyl)-5- methyl-2,4-dioxo-5-oxazolidinecarboxyate;chlozolinate (ISO) ethyl (RS)-3-(3,5-dichlorophenyl)-5-methyl-2,4-dioxo-oxazolidine-5-carboxylate;Chlozolinate solution;5-Oxazolidinecarboxylic acid, 3-(3,5-dichlorophenyl)-5-methyl-2,4-dioxo-, ethyl ester, (+-)- | | CAS: | 84332-86-5 | | MF: | C13H11Cl2NO5 | | MW: | 332.14 | | EINECS: | 282-714-4 | | Product Categories: | | | Mol File: | 84332-86-5.mol | 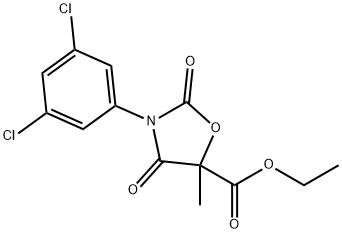 |
| | CHLOZOLINATE Chemical Properties |
| Melting point | 110-114 °C | | Boiling point | 420.8±55.0 °C(Predicted) | | density | 1.479 | | vapor pressure | 1.3 x 10-5 Pa (25 °C) | | Fp | 100 °C | | storage temp. | 0-6°C | | pka | -4.24±0.40(Predicted) | | Water Solubility | 2 mg l-1 (25 °C) |
| | CHLOZOLINATE Usage And Synthesis |
| Uses | Chlozolinate is a dicarboximide fungicide which is used primarily on grapes. | | Uses | Chlozolinate controls fruit rot, downy mildew and brown rot in
pome and stone fruits, vines, vegetables, strawberries and ornamentals
caused by Botrytis, Monilia and Sclerotinia, efc. | | Definition | ChEBI: Ethyl 3-(3,5-dichlorophenyl)-5-methyl-2,4-dioxo-1,3-oxazolidine-5-carboxylate is the ethyl ester of 3-(3,5-dichlorophenyl)-5-methyl-2,4-dioxo-1,3-oxazolidine-5-carboxylic acid. It is a dichlorobenzene, an oxazolidinone, a dicarboximide and an ethyl ester. | | General Description | Chlozolinate is a systemic fungicide, used for the control of downy mildew, brown rot, and fruit rot in agricultural commodities. | | Metabolic pathway | The fungicides, chlozolinate, vinclozolin, and
procymidone, are added to wine after fermentation
and the degradation products are isolated and
identified. Chlozolinate undergoes a rapid hydrolytic
loss of the ethoxycarbonyl substituent to give an
oxazolidine that further undergoes hydrolytic cleavage
to give 3' ,5' -dichloro-2-hydroxypropanilide. The
oxazolidine ring of vinclozolin undergoes a similar
hydrolysis reaction to give the corresponding anilide, 3' ,5'-dichloro-2-hydroxy-2-methylbut-3-eneanilide. Both
of these anilides are stable in wine for 150 days. A
different degradation behavior is observed with
procymidone and leads to the formation of 3,5-
dichloroaniline, which, in turn, breaks down in wine. | | Degradation | Chlozolinate (1) hydrolysed rapidly at 25 °C in alkaline solution with DTm
values of 6 hours (pH 6.8) and 16 min (pH 8.3). Three hydrolytic degradation
reactions were observed. The first reaction involved the cleavage of
the ethyl ester to yield the corresponding carboxylic acid (2). Compound
2 underwent decarboxylation to yield 3-(3,5-dichlorophenyl)-5-methyloxazolidine-
2,4-dione (3). The third reaction involved the opening of the
oxazolidinedione ring to yield 3',5'-dichloro-2-hydroxypropanilide(4)
(Villedieu et al., 1994,1995).
Chlozolinate degraded rapidly in wine during the vinification process
(DT50 0.35 and 0.14 day at pH 3 and 4, respectively). Decarboxylation
resulted in the formation of compound 3 as the major product (Cabras et
al., 1984; Pirisi et al., 1986; Gennari et al., 1992). |
| | CHLOZOLINATE Preparation Products And Raw materials |
|