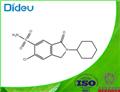
- clorexolone USP/EP/BP
-
- $1.10 / 1g
-
2021-08-10
- CAS:2127-01-7
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
|
| | clorexolone Basic information |
| Product Name: | clorexolone | | Synonyms: | 12833 RP;6-Chloro-2-cyclohexyl-2,3-dihydro-3-oxo-1H-isoindole-5-sulfonamide;Flonatril;M&B 8430;Nefrolan;6-chloro-2-cyclohexyl-3-keto-isoindoline-5-sulfonamide;6-chloro-2-cyclohexyl-3-oxo-1H-isoindole-5-sulfonamide;Chlorexolone | | CAS: | 2127-01-7 | | MF: | C14H17ClN2O3S | | MW: | 328.818 | | EINECS: | 2183426 | | Product Categories: | | | Mol File: | 2127-01-7.mol | 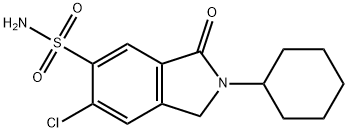 |
| | clorexolone Chemical Properties |
| Melting point | 266-268° | | storage temp. | Refrigerator | | solubility | DMSO (Slightly, Heated), Methanol (Very Slightly, Heated) | | form | Solid | | color | White to Off-White |
| | clorexolone Usage And Synthesis |
| Originator | Speciatensol,Specia,France,1966 | | Uses | Chlorexolone is a low-ceiling sulfonamide diuretic. | | Uses | Chlorexolone is a sulfonamide based antibiotic. | | Definition | ChEBI: Clorexolone is an organic molecular entity. | | Manufacturing Process | 4-Chlorophthalimide (263 g) was reacted in amyl alcohol (2.6 l) with
cyclohexylamine (143.5 g, 1 mol) at reflux temperature for 16 hours to give
N-cyclohexyl-4-chlorophthalimide (250 g, 66%) as a solid, MP 134°C to
136°C.
N-Cyclohexyl-1-chlorophthalimide (250 g) was dissolved in glacial acetic acid
(2.5 l), concentrated hydrochloric acid (555 ml) and tin (278 g) were added
and the suspension was heated on a steam bath for 16 hours. The cooled
solution was filtered and concentrated to dryness in vacuo to give a white
solid. This solid was dissolved in water and the precipitated oil extracted with
chloroform. The chloroform solution was dried and concentrated in vacuo to
give a solid which, after recrystallization, yielded 5-chloro-2-
cyclohexylisoindolin-1-one (43%), MP 140°C to 142°C.
5-Chloro-2-cyclohexylisoindolin-1-one (102.9 g) was dissolved in concentrated
sulfuric acid (665 ml); potassium nitrate (723 g) in concentrated sulfuric acid
(166 ml) was added at 0 °C. The reaction mixture was allowed to warm to
room temperature and stirred at 25°C for 12 hours. The reaction mixture was
poured onto ice to give a cream solid which, after recrystallization from
benzene, gave 5-chloro-2-cyclohexyl-6-nitroisoindolin-1-one (46.7 g, 44%) as
a white solid, MP 164°C to 168°C.
5-Chloro-2-cyclohexyl-6-nitroisoindolin-1-one (93.9 g) was reduced in
concentrated hydrochloric acid (1,970 ml) with stannous chloride (376 g). The
reaction temperature rose to 70°C. The resulting solution was cooled in ice
and filtered. The product was washed well with water, filtered and dried to
give 6-amino-5-chloro-2-cyclohexylisoindolin-1-one (74.1 g, 87.6%) which,
after recrystallization from benzene, had a MP of 216°C to 218°C.
6-Amino-5-chloro-2-cyclohexylisoindolin-1-one (42.5 g) was dissolved in
concentrated hydrochloric acid (425 ml) and the solution diazotized by the
addition of sodium nitrite (21.25 g) in water (125 ml). The resulting
diazonium salt solution was added to a solution of liquid sulfur dioxide (93 ml)
in glacial acetic acid (243 ml) containing cuprous chloride (2.25 g). A yellow
solid was precipitated; this was filtered off, washed, dried and recrystallized
from benzene to give 5-chloro-2-cyclohexylisoindolin-1-one-6-sulfonyl chloride
(45 g, 80%) as a cream solid, MP 171°C to 174°C.
This sulfonyl chloride (23.7 g) was reacted with liquid ammonia (237 ml) to
give 5-chloro-2-cyclohexyl-6-sulfamoylisoindolin-1-one (14.2 g, 53%). MP
259°C to 261°C. | | Therapeutic Function | Diuretic |
| | clorexolone Preparation Products And Raw materials |
|