|
|
| | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate Basic information |
| Product Name: | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate | | Synonyms: | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaMine (2R)-Hydroxy(phenyl)ethanoate;(αR)-α-Hydroxybenzeneacetic Acid coMpd. with (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaMine;trans-(1R,2S)-2-(2,3-difluorophenyl)cyclopropylaMine Mandelat;(alphaR)-alpha-Hydroxybenzeneacetic acid compd. with (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine;(αR)-(1R,2S)-α-hydroxy-Benzeneacetic acid-coMpd.with 2-(3,4-difluorophenyl)cyclopropanaMine(1:1);Ticagrelor InterMediate 4;(1R,2S)-2-(3,4-Difluorophenyl) cyclopropylamine D-Mandelate;(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaminium | | CAS: | 376608-71-8 | | MF: | C17H17F2NO3 | | MW: | 321.32 | | EINECS: | 1308068-626-2 | | Product Categories: | Intermediate of Ticagrelor;376608-71-8 | | Mol File: | 376608-71-8.mol | 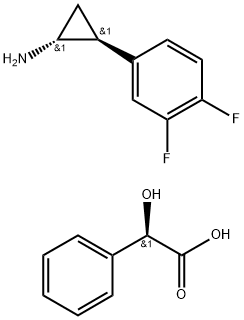 |
| | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate Chemical Properties |
| Melting point | 161 °C | | storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Off-White | | Major Application | Pharmaceutical intermediates | | InChI | InChI=1/C9H9F2N.C8H8O3/c10-7-2-1-5(3-8(7)11)6-4-9(6)12;9-7(8(10)11)6-4-2-1-3-5-6/h1-3,6,9H,4,12H2;1-5,7,9H,(H,10,11)/t6-,9+;7-/s3 | | InChIKey | GUESUQPLVFMJIT-RUKVIUEPNA-N | | SMILES | [C@H](C1C=CC=CC=1)(O)C(=O)O.N[C@@H]1C[C@H]1C1C=CC(F)=C(F)C=1 |&1:0,12,14,r| |
| Safety Statements | 24/25 | | HS Code | 29181990 |
| | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate Usage And Synthesis |
| Description | (1R, 2R)-2-(3, 4-difluorophenyl) cyclopropanamine(S)-(carboxylato(phenyl)methyl) holmium is a useful pharmacological intermediate. It is utilized as an intermediate of trcagrelor, which is a platelet aggregation inhibitor used for prevention of thrombotic events occurring in people with acute coronary syndrome or myocardial infarction. | | Chemical Properties | White to off-white powder | | Uses | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine is an intermediate in the preparation of orally active reversible P2Y12 receptor antagonists for the prevention of thrombosis. | | Synthesis | 1. Trans-(1R,2S)-2-(3,4-difluorophenyl)-1-nitro cyclopropane (215.0 g) was
added to the pre-cooled methanolic hydrochloric acid (6.0% to 7% w/w
HCl, 4300 ml), followed by cooling the mass to -5 to 0° C. Zinc dust
(343.71 g) was added to the resulting mass over a period of 2 to 3 hours
while maintaining the temperature at -5 to 0° C. The reaction mass was
stirred further for 2 hours at -5 to 0° C. After completion of the
reaction, the reaction mass was filtered through a hyflo bed and the bed
was washed with methanol (2×215 ml). The main filtrate and washings
were combined, followed by distillation under reduced pressure.
2. The
resulting residue was dissolved in dichloromethane (1075 ml) and then
cooled to 10 to 15° C. 25% Aqueous ammonia solution (1290 ml) was added
to the cooled solution while maintaining the temperature at below 30° C.
The resulting reaction mass was stirred for 15 minutes, followed the by
layer separation. The resulting aqueous layer was extracted with
dichloromethane (2×537.5 ml) and then combined with the main
dichloromethane layer. The combined dichloromethane layer containing the
product was extracted thrice with aqueous hydrochloric acid (645 ml of
conc. hydrochloric acid mixed with 1935 ml water, 3×865 ml). The aqueous
acidic layer containing the product was combined and washed with
dichloromethane (645 ml). Dichloromethane (1075 ml) was added to the
acidic aqueous layer, followed by the addition of 25% aqueous ammonia
solution (1505 ml) while maintaining the temperature at below 30° C. The
resulting reaction mass was extracted with dichloromethane (2×645 ml)
and then combined with the main dichloromethane layer. The combined
dichloromethane layer containing the product was washed with water (645
ml) and evaporated to dryness under reduced pressure.
3. The resulting
residue was dissolved in methanol (430 ml), followed slow addition of
(R)-(-)-mandelic acid solution (107.5 g in 645 ml methanol) over a
period of 40 to 60 minutes while maintaining temperature at 20 to 25° C.
The resulting slurry was stirred further for 12 hours at 20 to 25° C.,
followed by further cooling to 0 to 5° C. The cooled solution was
stirred for 2 hours and the solid was isolated by filtration. The
resulting solid was washed with chilled methanol (215 ml). The solid was
dried under reduced pressure at 40 to 45° C. to obtain 127 g of pure (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate as a white solid. | | References | http://www.tradekorea.com/product/detail/P483269/Ticagrelor-intermediates---CAS-376608-71-8.html
https://en.wikipedia.org/wiki/Ticagrelor |
| | (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate Preparation Products And Raw materials |
|