- CUCURBITACIN I
-

- $1.00 / 1KG
-
2019-07-06
- CAS:2222-07-3
- Min. Order: 1G
- Purity: 98%
- Supply Ability: 100KG
|
| | CUCURBITACIN I Basic information |
| Product Name: | CUCURBITACIN I | | Synonyms: | ELATERICIN B;ELATERIN B;CUCURBITACIN I WITH HPLC;Cucurbitacin I hydrate;Elatericin B, JSI-124, NSC 521777, 2,16α,20,25-Tetrahydroxy-9-methyl-19-Nor-9β,10α-lanosta-1,5,23-triene-3,11,22-trione;(9β,10α,23E)-2,16α,20,25-Tetrahydroxy-9-methyl-19-norlanosta-1,5,23-triene-3,11,22-trione;Ibamarin;2,16a,20,25-Tetrahydroxy-9b-methyl-10a-19-norlanosta-1,5,23(E)-triene-3,11,22-trione | | CAS: | 2222-07-3 | | MF: | C30H42O7 | | MW: | 514.65 | | EINECS: | 218-736-8 | | Product Categories: | Inhibitor;Herb extract;API;Tri-Terpenoids | | Mol File: | 2222-07-3.mol | 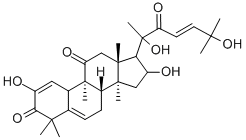 |
| | CUCURBITACIN I Chemical Properties |
| Melting point | 148-150°C | | Boiling point | 698.3±55.0 °C(Predicted) | | density | 1.26±0.1 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | ≥22.45 mg/mL in DMSO; insoluble in EtOH; ≥51.2 mg/mL in H2O with ultrasonic | | pka | 8.51±0.70(Predicted) | | form | solid | | color | white to off-white | | LogP | 2.330 (est) |
| Hazard Codes | Xi,T+ | | Risk Statements | 25-28 | | Safety Statements | 1-22-45-36/37-28 | | RIDADR | UN 2811 6.1/PG 1 | | WGK Germany | 3 | | RTECS | RC6200000 | | Toxicity | LD50 oral in mouse: 5mg/kg |
| | CUCURBITACIN I Usage And Synthesis |
| Uses | Cucurbitacin I can be useful in the study of edible vitalmelon fruit extract and adipogenesis. | | Definition | ChEBI: A cucurbitacin that is 9,10,14-trimethyl-4,9-cyclo-9,10-secocholesta-2,5,23-triene substituted by hydroxy groups at positions 2, 16, 20 and 25 and oxo groups at positions 1, 11 and 22. | | Biological Activity | Selective inhibitor of STAT3/JAK2 signaling. Inhibits the activation of STAT3 and JAK2 and displays no activity on Src, Akt, ERK and JNK. Suppresses phosphotyrosine levels of STAT3, inhibits STAT3 DNA binding and STAT3-mediated gene expression. Induces apoptosis in cell lines expressing constitutively active tyrosine-phosphorylated STAT3. | | Biochem/physiol Actions | Cucurbitacin I (JSI-124) is a novel selective inhibitor of the janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway with anti-proliferative and anti-tumor properties. | | storage | Store at -20°C | | references | blaskovich ma, sun j, cantor a et al. discovery of jsi-124 (cucurbitacin i), a selective janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice.cancer res. 2003 mar 15;63(6):1270-9.yu h, lee h, herrmann a et al. revisiting stat3 signalling in cancer: new and unexpected biological functions.nat rev cancer. 2014 nov;14(11):736-46. doi: 10.1038/nrc3818.song j, liu h, li z et al. cucurbitacin i inhibits cell migration and invasion and enhances chemosensitivity in colon cancer. oncol rep. 2015 apr;33(4):1867-71. qi j, xia g, huang cr et al. jsi-124 (cucurbitacin i) inhibits tumor angiogenesis of human breast cancer through reduction of stat3 phosphorylation. am j chin med. 2015;43(2):337-47.kim hj, kim jk et al. antiangiogenic effects of cucurbitacin-i. arch pharm res. 2015 feb;38(2):290-8. yuan g, yan sf, xue h et al. cucurbitacin i induces protective autophagy in glioblastoma in vitro and in vivo. j biol chem. 2014 apr 11;289(15):10607-19. |
| | CUCURBITACIN I Preparation Products And Raw materials |
|