| Company Name: |
Jinan ponder chemical co. LTD
|
| Tel: |
0531-0000 |
| Email: |
thinklifescience@163.com |
| Products Intro: |
Product Name:123482-22-4
CAS:123482-22-4
Purity:99% HPLC Package:1KG;500G;100G;50G;1G
|
| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
15002134094 |
| Email: |
marketing@targetmol.com |
| Products Intro: |
Product Name:Zatosetron
CAS:123482-22-4
Purity:详情请点击官网 Package:1 mg
|
|
| | Zatosetron Basic information |
| Product Name: | Zatosetron | | Synonyms: | Zatosetron;5-Chloro-2,3-dihydro-2,2-dimethyl-N-[(1β,5β)-8-methyl-8-azabicyclo[3.2.1]octan-3α-yl]benzofuran-7-carboxamide;LY-277359;123482-23-5 (Maleate);7-Benzofurancarboxamide, 5-chloro-2,3-dihydro-2,2-dimethyl-N-(8-methyl-8-azabicyclo(3.2.1)oct-3-yl)-, endo-;Zatosetron [inn:ban];Zatosetronum;Zatosetronum [inn-latin] | | CAS: | 123482-22-4 | | MF: | C19H25ClN2O2 | | MW: | 348.87 | | EINECS: | | | Product Categories: | | | Mol File: | 123482-22-4.mol | 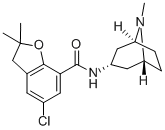 |
| | Zatosetron Chemical Properties |
| | Zatosetron Usage And Synthesis |
| Originator | Zatosetron
maleate,Eli-Lilly | | Uses | Antimigraine. | | Manufacturing Process | A mixture of 5-chloromethyl salicylate, 3-chloro-2-methylpropene, potassium
carbonate, and acetone was heated at reflux overnight. After cooling, the
mixture was extracted with diethyl ether and ethyl acetate. The organic
extracts were combined, washed twice with a 10% sodium chloride solution
and water, dried over sodium sulfate, and concentrated in vacuum. The
resulting liquid was vacuumed distilled. The fraction collected and the desired
5-chloro-2-(2-methyl-2-propenyloxy)benzoic acid, methyl ester was obtained.
The 5-chloro-2-(2-methyl-2-propenyloxy)benzoic acid, methyl ester was
heated at reflux for 6 h in 1-methyl-2-pyrrolidinone. The mixture was then
vacuum distilled and the fraction collected and the desired 2-hydroxy-5-
chloro-3-(2-methyl-2-propenyl)benzoic acid, methyl ester was obtained.
A mixture of the 2-hydroxy-5-chloro-3-(2-methyl-2-propenyl)benzoic acid,
methyl ester and 1 L of methanol was saturated with 90% formic acid and
then refluxed overnight. The solution was concentrated in vacuum, added to
water, and extracted with ethyl acetate. The organic layer was washed with
water, dried over sodium sulfate, and concentrated in vacuum, providing the
desired 2,2-dimethyl-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid,
methyl ester as an oil.
The 2,2-dimethyl-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid, methyl
ester were heated at reflux with sodium hydroxide and water for 2-3 h. After
cooling, the mixture was extracted with diethyl ether and ethyl acetate. The
aqueous layer was acidified with hydrochloric acid and again extracted with
ethyl acetate and diethyl ether. These latter organic extracts were combined
and washed with water, dried over sodium sulfate, and concentrated in
vacuum. Crystallization of the resulting solid from ethyl acetate/hexane
provided the desired 2,2-dimethyl-5-chloro-2,3-dihydrobenzofuran-7-
carboxylic acid, 71% yield, melting point 158.5°-160°C.
A mixture of 2,2-dimethyl-5-chloro-2,3-dihydrobenzofuran-7-carboxylic acid
and thionyl chloride was heated at reflux for 3 h. After the mixture was
3522 Zatosetron maleate
concentrated in vacuum and azeotroped with toluene, dry toluene was added
and the solution cooled to 5°C. A solution of N-methyl-tropamine in toluene
was added in dropwise fashion and the reaction heated at reflux overnight.
After cooling, the mixture was added to ice water, made basic, and extracted
with diethyl ether/ethyl acetate. The organic layer was washed twice with 6 N
hydrochloric acid. The combined aqueous extracts were cooled, made basic
with sodium hydroxide solution, and extracted with ethyl acetate. The ethyl
acetate solution was washed twice with water, dried over sodium sulfate, and
concentrated in vacuum providing of the endo-5-chloro-2,3-dihydro-2,2-
dimethyl-N-(8-methyl-8-azabicyclo[3.2.1.]oct-3-yl-7-benzofurancarboxamide,
free base, as an oil.
In practice it is usually used as maleate salt. | | Therapeutic Function | Serotonin antagonist |
| | Zatosetron Preparation Products And Raw materials |
|