| | Nitric acid Basic information |
| Product Name: | Nitric acid | | Synonyms: | acidenitrique;AZOTIC ACID;AQUA FORTIS;CONTRACT LAB PROGRAM AA/ICP NITRIC ACID REAGENT/MATRIX BLANK SOLUTION;ELUENT (1MMOL/L HNO3);Nitric acid, 70%, redistilled, 99.999+% metals basis;NITRIC ACID 70% SEMICONDUCTOR GRADE PURA;NITRIC ACID MIN. 65 %, R. G., REAG. REAG. ISO, REAG. PH. EUR., FOR DET. WITH DIT | | CAS: | 7697-37-2 | | MF: | HNO3 | | MW: | 63.01 | | EINECS: | 231-714-2 | | Product Categories: | Semiconductor Grade Chemicals;Synthetic Reagents;Acids, Mercury-free and Arsenic-freeAnalytical Reagents for General Use;I-N, Puriss p.a.;Atomic Absorption Spectroscopy (AAS);Digestion Reagents;Puriss p.a.;CO - CZSpectroscopy;MultielementSpectroscopy;AA/ICP Standard SolutionsAlphabetic;AAS;C;Matrix Selection;Other;Reference/Calibration Standards;Standard Solutions;Analytical/Chromatography;TraceSelectUltraAtomic Absorption Spectroscopy (AAS);Ultrapure Reagents for Wet Digestion (Trace SELECTUltra);Chromogenic&Charring agents (general)Analytical Reagents for General Use;I-N, Puriss p.a.Derivatization Reagents TLC;Derivatization Reagents;Derivatization Reagents TLC;TLC Reagents, N-R;TLC Visualization Reagents (alphabetic sort);M-N, Puriss p.a. ACS;Puriss p.a. ACS;ACS GradeChemical Synthesis;Acid SolutionsVolumetric Solutions;Essential Chemicals;Reagent Grade;AcidsChemical Synthesis;Micro/Nanoelectronics;Analytical Reagents for General Use;Inorganic AcidsAnalytical Reagents;Puriss;Acid ConcentratesConcentrates (e.g. FIXANAL);By Reference Material;Inorganic AcidsVolumetric Solutions;Eluent concentrates for ICChromatography/CE Reagents;Chromatography/CE Reagents;HPLC Buffer;Ion Chromatography;Solution;Inorganics;Acid SolutionsChemical Synthesis;Concentrates (e.g. FIXANAL);Reference Material Sodium carbonate;Acids&Bases;Analytical Reagents;Pure Reagents for Wet Digestion (Trace SELECT)Analytical/Chromatography;Trace Analysis ReagentsEssential Chemicals;TraceSelect;Acid Solutions;Titration;Volumetric Solutions;Inorganic Acids;Chemical Synthesis;Electronic Chemicals;Inorganic AcidsMicro/Nanoelectronics | | Mol File: | 7697-37-2.mol | 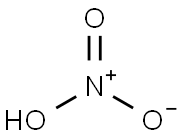 |
| | Nitric acid Chemical Properties |
| Melting point | -42 °C | | Boiling point | 120.5 °C(lit.) | | density | 1.41 g/mL at 20 °C | | vapor density | 1 (vs air) | | vapor pressure | 8 mm Hg ( 20 °C) | | Fp | 120.5°C | | storage temp. | Store at +2°C to +25°C. | | solubility | Miscible with water. | | pka | -1.3(at 25℃) | | form | Liquid, Double Sub-Boiling Quartz Distillation | | Specific Gravity | d 20/4 1.4826 | | color | colorless to deep yellow | | PH Range | 1 | | Odor | Suffocating fumes detectable at <5.0 ppm | | PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution); | | Water Solubility | >100 g/100 mL (20 ºC) | | Sensitive | Hygroscopic | | Merck | 14,6577 | | Exposure limits | TLV-TWA 2 ppm (5 mg/m3) (ACGIH,
MSHA, OSHA, and NIOSH); STEL 4 ppm
(~10 mg/m3) (ACGIH). | | Dielectric constant | 55.0(14℃) | | LogP | -0.773 (est) | | CAS DataBase Reference | 7697-37-2(CAS DataBase Reference) | | NIST Chemistry Reference | Nitric acid(7697-37-2) | | EPA Substance Registry System | Nitric acid (7697-37-2) |
| | Nitric acid Usage And Synthesis |
| Description | Nitric acid is a colorless, corrosive liquid that is the most common nitrogen acid. It has been used for hundreds of years. Nitric acid is a mineral acid that was called spirit of nitre and aqua fortis, which means strong water.

Fuming nitric acid is named because of the fumes emitted by acid when it combines with moist air. Fuming nitric acid is highly concentrated and is labeled either red fuming nitric acid or white fuming nitric acid. Red fuming nitric acid, as the name implies, emits a reddishbrown fume on exposure to air. The color comes from nitrogen dioxide, which is liberated on exposure to air. The nitric acid concentration of red fuming nitric acid is approximately 85% or greater, with a substantial amount of dissolved nitrogen dioxide. White fuming nitric acid is highly concentrated anhydrous nitric acid with concentrations of 98–99%; the remaining 1–2% is water and nitrogen dioxide. Most commercial grade nitric acid has a concentration of between 50% and 70%. | | Chemical Properties | Nitricacid,HN03, is a strong,fire-hazardous oxidant. It is a colorless or yellowish liquid that is miscible with water and boils at 86℃ (187 ℉). Nitric acid, also known as aqua fortis, is used for chemical synthesis, explosives, and fertilizer manufacture, and in metallurgy, etching, engraving, and ore flotation. | | Chemical Properties | Nitric acid is a colorless to light brown fuming
liquid with an acrid, suffocating odor. Fuming nitric
acid is a reddish fuming liquid. Fumes in moist air. Often
used in an aqueous solution. Fuming nitric acid is concentrated
nitric acid that contains dissolved nitrogen dioxide.
Nitric acid is a solution of nitrogen dioxide, NO2, in water
and so-called fuming nitric acid contains an excess of NO2
and is yellow to brownish-red in color. | | Physical properties | Colorless liquid; highly corrosive; refractive index 1.397 at 16.5°C; density 1.503 g/L; freezes at –42°C; boils at 83°C; completely miscible with water; forms a constant boiling azeotrope with water at 68.8 wt% nitric acid; the azeotrope has density 1.41 g/mL and boils at 121°C. | | History | Nitric acid was known to alchemists in ancient times. Cavendish in 1784 synthesized the acid by applying an electric spark to humid air. Earlier in 1776, Lavoisier determined that the acid contained oxygen. In 1798, Milner prepared nitric acid from ammonia along with nitrogen oxides by oxidation of ammonia vapor over red-hot manganese dioxide. In 1816, Gay-Lussac and Berthollet established its composition.
Nitric acid is one of the most important industrial chemicals in the world. Its largest use is in the fertilizer industry for producing various nitrate fertilizers. Such fertilizers include ammonium-, sodium-, potassium-, and calcium nitrates. Other major applications of nitric acid are in making nitrates and nitrooganics for use in explosives, gunpowder, and fireworks. Ammonium nitrate, nitroglycerine, nitrocellulose, and trinitrotoluenes are examples of such explosives, while barium and strontium nitrates are used in fireworks. NITRIC ACID 635Another major application is in producing cyclohexanone, a raw material for adipic acid and caprolactam to produce nylon.
Nitric acid is a common laboratory reagent. It also is one of the most used oxidizing agents, applied in several organic and inorganic syntheses. Some synthetic applications of nitric acid include the production of diazo dyes, varnishes, lacquers, plastics, polyurethanes, and detergents. Other applications are in metal etching, ore extractions, pickling of stainless steel, rocket propellant, for processing nuclear fuel, as a solvent in aqua regia, for sample digestion in metal analysis by AA or ICP, and in preparing analytical standards.
Concentrated nitric acid used in commerce is not 100% pure nitric acid. It is the constant boiling mixture containing 68% pure acid. | | Uses | Nitric acid is an important starting material for the production of fertilizers and chemicals. Diluted nitric acid is used for dissolving and etching metals Product Data Sheet | | Uses | The main use of nitric acid is for the production of fertilizer, with approximately threefourthsof nitric acid production being used for this purpose. Ammonium nitrate is the preferred nitrogen fertilizer owing to itsease in production, economics, and high nitrogen content, which is 35%. Nitric acid can alsobe used for the acidulation of phosphate rock to produce nitrogen-phosphorus fertilizers.Nitric acid is a strong oxidizer, which makes it useful in explosives and as a rocketpropellant.
Nitric acid is used for nitrating numerous other compounds to produce nitrates. Nitricacid is used to produce adipic acid (C6H4O10), which is used in the production of nylon (seeNylon).
Additional uses of nitric acid are for oxidation, nitration, and as a catalyst in numerousreactions. Salts of nitric acid are collectively called nitrates, which are soluble in water. Nitricacid is used in the production of many items such as dyes, pharmaceuticals, and syntheticfabrics.It is also used in a variety of processes including print making.
Nitric acid is used extensively in the metal industries. Nitric acid is used to pickle steel and brass surfaces in metal processing. | | Uses | Nitric acid is an important material for the production of explosives. Concentrated nitric acid, usually mixed with sulfuric acid (mixed acid), is used for nitrating organic compounds. Product Data Sheet | | Uses | This heavy, clear or slightly yellowish fluid is very poisonous
and causes severe burns on contact with the skin. It was made
by the distillation of an alkali-metal nitrate combined with
sulfuric acid. The combination of nitric and sulfuric acids was
used to convert plain cotton to cellulose nitrate. Nitric acid
was used in the wet plate process as an additive to ferrous sulfate developers to promote a whiter image color for ambrotypes
and ferrotypes. It was also added to lower the pH of the
silver bath for collodion plates. Adding acid to the silver bath
made collodion plates less sensitive to light, which had the
beneficial effect of reducing the occurrence non-image fog. | | Uses | Nitric acid is one of the most widely usedindustrial chemicals. It is employed in the production of fertilizers, explosives, dyes, synthetic fibers, and many inorganic and organicnitrates; and as a common laboratory reagent.. | | Uses | Nitric acid (HNO3) is an important industrial acid used to alter or produce many products
such as fertilizers and explosives. It reacts with ammonia to produce ammonium nitrate, an
important commercial chemical. | | Definition | nitric acid: A colourless corrosivepoisonous liquid, HNO3; r.d. 1.50;m.p. –42°C; b.p. 83°C. Nitric acid maybe prepared in the laboratory by thedistillation of a mixture of an alkalimetalnitrate and concentratedsulphuric acid. The industrial productionis by the oxidation of ammoniato nitrogen monoxide, theoxidation of this to nitrogen dioxide,and the reaction of nitrogen dioxidewith water to form nitric acid and nitrogenmonoxide (which is recycled).The first reaction (NH3 to NO) iscatalysed by platinum or platinum/rhodium in the form of fine wiregauze. The oxidation of NO and theabsorption of NO2 to form the productare noncatalytic and proceedwith high yields but both reactionsare second-order and slow. Increasesin pressure reduce the selectivity ofthe reaction and therefore ratherlarge gas absorption towers are required.In practice the absorbing acidis refrigerated to around 2°C and acommercial ‘concentrated nitric acid’at about 67% is produced.Nitric acid is a strong acid (highlydissociated in aqueous solution) anddilute solutions behave much likeother mineral acids. Concentrated niniobium tric acid is a strong oxidizing agent.
Most metals dissolve to form nitratesbut with the evolution of nitrogenoxides. Concentrated nitric acid alsoreacts with several nonmetals to givethe oxo acid or oxide. Nitric acid isgenerally stored in dark brown bottlesbecause of the photolytic decompositionto dinitrogen tetroxide. Seealso nitration. | | Production Methods | Nitric acid may be produced by several methods. In the laboratory, it is prepared by distilling a solution of potassium nitrate in concentrated sulfuric acid containing equal amounts (by weight) of each.
KNO3 + H2SO4 → KHSO4 + HNO3
Nitric acid decomposes to nitrogen dioxide. Therefore, the temperature must be kept as low as possible. During this preparation, nitric acid condenses as a fuming liquid. The pure acid may be obtained when it is collected at –42°C, its freezing point. When nitric acid is collected by condensation at room temperature, it may decompose partially to nitrogen pentaoxide, N2O5, which fumes in moist air. Early commercial processes were based on reaction of Chile saltpeter (NaNO3) with sulfuric acid. Concentrated nitric acid was obtained by distilling the reaction mixture.
Nitric acid also may be obtained by rapid passage of air through an electric 636 NITRIC ACIDarc. The method is based on Cavendish’s first preparation of nitric acid. In this method, nitrogen and oxygen first combine to form nitric oxide. The gaseous product mixture usually containing about 2% nitric oxide is combined with excess oxygen to form nitrogen dioxide and nitrogen pentoxide. Dissolution of these gases in water forms nitric acid. The process, however, is expensive and unsuitable for commercial application.
Currently, nitric acid is manufactured exclusively by catalytic oxidation of ammonia. Platinum or platinum-rhodium is an effective catalyst of this oxidation (Ostwald process). Three basic steps in such ammonia oxidation process are: (1) oxidation of ammonia to form nitric oxide:
4NH3 + 5O2 → 4NO + 6H2O
The above reaction is rapid and shifts almost fully to the product side. (2) oxidation of nitric oxide to form nitrogen dioxide:
2NO + O2 → 2NO2
The above reaction also is rapid and goes almost to completion below 150°C. (3) dissolution of nitrogen dioxide in water:
3NO2 + H2O → 2HNO3 + NO
This reaction is moderately exothermic, releasing 32.4 kcal/mol.
Several mechanisms have been proposed for absorption of nitrogen dioxide in water. Nitrogen dioxide readily dimerizes to tetroxide, N2O4, at low temperatures and increasing pressure.
2NO2 ↔ N2O4 ?Hrxn = –13.7 kcal/mol
Absorption of tetroxide in water also could form nitric acid and nitric oxide:
3N2O4 + 2H2O → 4HNO3 + 2NO
Several modifications in plant design and process conditions for ammonia oxidation processes have taken place in recent years. These variations are more or less based on operating pressures and temperatures, reduction of NOx emission and other environmental regulations, and the desired plant production capacity.
Nitric acid obtained in standard ammonia oxidation is usually 50 to 70% by weight aqueous solution. Pure nitric acid of 98-99% may be obtained either by extractive distillation or by direct strong nitric (DSN) processes. In the distillation method, concentrated nitric acid of 50-70% is distilled with 93% sulfuric acid in a steam-heated tower. Sulfuric acid acts as a dehydrating agent. The distilled nitric acid vapor is condensed to pure nitric acid, while sulfuric acid absorbing water from 50-70% nitric acid loses its strength to about 70% and collects at the bottom. The 70% sulfuric acid is concentrated back to 93% NITRIC ACID 637for reuse by removal of water in a sulfuric acid concentrator.
In the DSN process, nitrogen tetroxide, N2O4 obtained from ammonia oxidation is absorbed by concentrated nitric acid in the presence of air or oxygen to yield pure nitric acid. Alternatively, N2O4 may be separated from the product gases of the ammonia oxidation process by refrigeration and then is treated with dilute nitric acid in air or oxygen. | | General Description | Nitric acid is a colorless to yellow or red liquid sometimes fuming reddish brown vapors with a suffocating odor. Nitric acid is soluble in water with release of heat. Nitric acid is corrosive to metals or tissue. Nitric acid will accelerate the burning of combustible materials and Nitric acid may even cause ignition upon contact with combustible material. Nitric acid is fully soluble in water and may react violently upon contact with water with the evolution of heat, fumes and spattering. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Density 10.4 lb / gal. | | Air & Water Reactions | Fumes in air. Fully soluble in water with release of heat. Reacts violently with water with the production of heat, fumes, and spattering. | | Reactivity Profile | Nitric acid; ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. The reaction of finely divided antimony and nitric acid can be violent [Pascal 10:504. 1931-34]. Bromine pentafluoride reacts violently with strong nitric acid and strong sulfuric acid [Mellor 2, Supp. 1:172. 1956]. Experiments show that mixtures of over 50% nitric acid by weight in acetic anhydride may act as detonating explosives [BCISC 42:2. 1971]. An etching agent of equal portions of acetone, nitric acid, and 75% acetic acid exploded 4 hours after Nitric acid was prepared and placed in a closed bottle. This is similar to a formulation for the preparation of tetranitromethane a sensitive explosive [Chem. Eng. News 38: 56. 1960]. Phosphine is violently decomposed by concentrated nitric acid, and flame is produced. Warm fuming nitric acid, dropped in a container of phosphine gas produces an explosion [Edin. Roy. Soc. 13:88. 1835]. An explosion occurs when nitric acid is brought into contact with phosphorus trichloride [Comp. Rend. 28:86]. The reaction of sodium azide and strong nitric acid is energetic [Mellor 8, Supp 2:315. 1967]. Reacts violently with water with the production of heat, fumes, and spattering. | | Hazard | Because it is a strong oxidizing agent, nitric acid may undergo violent reactions with powerful reducing agents. Many nitration reactions of organics yield explosive products. Pure nitric acid is highly corrosive to skin causing severe injury. Concentrated acid (68.8 wt %) is moderately corrosive to skin. The acid may decompose under heating or photochemically, liberating toxic nitrogen dioxide gas. | | Health Hazard | Nitric acid is a corrosive substance causingyellow burns on the skin. It corrodes the bodytissues by converting the complex proteinsto a yellow substance called xanthoproteicacid (Meyer 1989). Ingestion of acid canproduce burning and corrosion of the mouthand stomach. A dose of 5–10 mL can befatal to humans.
Chronic exposure to the vapor and mist ofnitric acid may produce bronchitis and chemical pneumonitis (Fairhall 1957). It emitsNO2, a highly toxic gas formed by its decomposition in the presence of light. Nitric acidis less corrosive than sulfuric acid. Its vaporand mist may erode teeth.. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. | | Flammability and Explosibility | Explosibility Not a combustible substance, but a strong oxidizer. Contact with easily
oxidizible materials including many organic substances may result in fires or
explosions. | | Agricultural Uses | Nitric acid (HNO) is a mineral acid which combines with
metals or alkalis to form nitrates. It can be made
synthetically by passing ammonia and air over a metallic
catalyst.
Nitric acid is used in the manufacture of ammonium
nitrate for explosives and fertilizers. Two other types of
nitric acid - red fuming nitric acid and white fuming
nitric acid are known. White fuming nitric acid contains more than 97.5 % nitric acid, less than 2 % water and less
than 0.5% oxides of nitrogen. Red fuming nitric acid
contains more than 86% nitric acid, 6 to 15% oxides of
nitrogen and less than 5 % water. It is used as a rocket fuel
and nitrating agent. Important production process for the
manufacture of nitric acid are elaborated in the entry
Nitric acid production processes.
Nitric acid production processes2
About 75% of nitric acid produced in the world is used
for producing fertilizers (and about 15% for explosives).
The remaining acid is used in making synthetic fibers,
dyes and plastic.
Nitric acid can be produced by (a) acidulation of
natural sodium nitrate with sulphuric acid, (b) direct
synthesis from nitrogen and oxygen, and (c) oxidation of
ammonia.
Nitric acid is a strong acid and a powerful oxidizing
agent. Concentrated nitric acid on distillation at
atmospheric pressure starts boiling at 78.2℃ but
decomposes eventually to give 68% nitric acid with a
boiling point of 120.5℃. The standard limit of chlorine is
less than 5 ppmw and that of nitrous acid (HNO2) less
than 5 ppmw. Anhydrous nitric acid does not exist in
liquid form.
Anhydrous ammonia and a platinum catalyst are
required for the manufacture of nitric acid. The oxides of
nitrogen that are used in the production of nitric acid are
nitrous oxide (N2O), nitric oxide (NO), nitrogen dioxide
(NO2) and dinitrogen tetroxide (N2O4). A mixture of
nitrogen oxides, usually NO and NO2, is commonly
referred to as NOx.
The production of weak nitric acid consists of the
following three steps: (a) catalytic ammonia oxidation to nitric oxide, (b) oxidation of nitric oxide to nitrogen I
dioxide, and (c)acidic absorption of nitrogen dioxide in
water.
The absorber performance is improved by high
pressure and low temperature, and a high oxygen content
in the gas phase. However, in the ammonia converter, the
oxidation of ammonia is favored by low pressure.
Methods of production: Many processes for
producing nitric acid are now available. They differ not
in fundamental principals, but primarily in design details
of the plant, operating conditions for the plant size, cost
considerations relating to raw materials, energy and
installation.
The production of nitric acid by the oxidation of
ammonia goes through the following steps or units: (a)
ammonia preparation-vaporization, superheating and
filtration of anhydrous ammonia, (b) process air
preparation involving preheating, filtration and
compression, (c) catalytic ammonia oxidation, (d)
cooling of the reaction products with various media such
as process air, boiler water, tail gas, etc., (e) oxidation of
nitric oxide to higher oxides, (f) nitrogen oxides
absorption in water to form nitric acid, (g) acid bleaching
by additional air or other means, (h) tail gas treatment to
reduce air pollution and to improve overall efficiency of
the plant, (i) recovery of energy from the heated and
compressed process gases, and (i) recovery of catalyst
platinum.
The anhydrous ammonia and the process air used
must be free both from the oil content and catalyst
poisons to avoid fouling of the vaporizer and catalyst
screens. The ratio of ammonia to air and the flow rate of
each component must be carefully controlled to ensure
maximum conversion efficiency, explosion prevention
and plant output maximization.
The normal catalyst used in the process is a platinumrhodium
gauze or mesh. It not only promotes reaction but
also meets the other operating criteria such as those to
counter the severe corrosive and oxidation atmosphere,
etc. The usual rhodium percentage in the catalyst is in the
range of 4 to 10%. To achieve higher efficiencies and
smaller platinum losses, knitted gauzes are used.
Chrome-nickel alloy grids are used to support the fine
screens.
Platinum catalysts can be poisoned by such elements
as As, Bi, P, Pb, S, Si and Sn. These elements lead to the
formation of inactive compounds in the wires and thus
decrease the catalytic activity. In some instances
contamination with traces of Cr, Ni or Fe temporarily
reduces the conversion efficiency which can often be
restored by treatment with hydrochloric acid. | | Industrial uses | Also called aqua fortis and azotic acid, nitricacid is a colorless to reddish fuming liquid ofthe composition HNO3, having a wide varietyof uses for pickling metals, etching, and in themanufacture of nitrocellulose, plastics, dyestuffs,and explosives. It has a specific gravityof 1.502 (95% acid) and a boiling point of 86°C,and is soluble in water. Its fumes have a suffocatingaction, and it is highly corrosive andcaustic. Fuming nitric acid is any water solutioncontaining more than 86% acid and having aspecific gravity above 1.480. Nitric acid is madeby the action of sulfuric acid on sodium nitrateand condensation of the fumes. It is also madefrom ammonia by catalytic oxidation, or fromthe nitric oxide produced from air. | | Safety Profile | Poison by inhalation. A
corrosive irritant to skin, eyes, and mucous
membranes. A very dangerous fire hazard
and very powerful oxidizing agent. Can react
explosively with many reducing agents. Wdl
react with water or steam to produce heat
and toxic, corrosive, and flammable vapors.When heated to decomposition it emits
hghly toxic fumes of NOx. See also
NITRIC ACID. | | Safety | Nitric acid is used in the manufacture of ammonium nitrate fertilizer and explosives, in steel etching, and in reprocessing spent nuclear fuel. There are two types of fuming nitric acid. White fuming nitric acid is concentrated with 97.5% nitric acid and less than 2% water. It is a colorless to pale-yellow liquid that fumes strongly. It is decomposed by heat and exposure to light and becomes red in color from nitrogen dioxide. Red fuming nitric acid contains more than 85% nitric acid, 6%–15% nitrogen dioxide, and 5% water. The four-digit UN identification number for red fuming nitric acid is 2032. The NFPA 704 designation is health 4, flammability 0, and reactivity 1. The prefix “oxy” appears in the white section of the diamond. Red fuming nitric acid is considered an oxidizer. Both white and red fuming acids are toxic by inhalation, strong corrosives, and dangerous fire risks that may explode upon contact with reducing agents. They are used in the production of nitro compounds, rocket fuels, and as laboratory reagents. | | Potential Exposure | Nitric acid is the second most important
industrial acid and its production represents the sixth
largest chemical industry in the United States. Nitric acid is
used in chemicals, explosives, fertilizers, steel pickling;
metal cleaning. The largest use of nitric acid is in the production
of fertilizers. Almost 15% of the production goes
into the manufacture of explosives, with the remaining
10% distributed among a variety of uses, such as etching,
bright-dipping; electroplating, photoengraving, production
of rocket fuel; and pesticide manufacture. | | Carcinogenicity | Nitric acid was not mutagenic in limited
studies.4 There is no information regarding the
carcinogenic properties of nitric acid, but an
association between incidences of laryngeal
cancer and exposure to acid mists has been
indicated.4 | | storage | Splash goggles and rubber gloves should be worn when handling
this acid, and containers of nitric acid should be stored in a well ventilated location separated
from organic substances and other combustible materials. | | Shipping | UN2031 Nitric acid other than red fuming, with
.70% nitric acid or Nitric acid other than red fuming,
with at least 65%, but not >70% nitric acid, Hazard Class:
8; Labels: 8-Corrosive material, 5.1-Oxidizer. UN2032
Nitric acid, red fuming, Hazard Class: 8; Labels:
8-Corrosive material, 5.1-Oxidizer, 6.1-Poisonous material.
Inhalation, Hazard Zone B. UN2031 Nitric acid other than
red fuming, with >20% and <65% nitric acid or Nitric
acid other than red fuming, with not >20% nitric acid,
Hazard Class: 8; Labels: 8-Corrosive material. | | Purification Methods | The acid is obtained colourless (approx. 92%) by direct distillation of fuming HNO3 under reduced pressure at 40-50o with an air leak at the head of the fractionating column. Store it in a desiccator kept in a refrigerator. Nitrite-free HNO3 can be obtained by vacuum distillation from urea. [Ward et al. Inorg Synth III 13 1950, Kaplan & Schechter Inorg Synth IV 53 1953.] | | Incompatibilities | A strong oxidizer and strong acid. Reacts
violently with combustible and reducing agents; carbides,
hydrogen sulfide, turpentine, charcoal, alcohol, powdered
metals; strong bases. Heat causes decomposition producing
nitrogen oxides. Attacks some plastics. Corrosive to metals. | | Waste Disposal | Soda ash-slaked lime is added
to form the neutral solution of nitrate of sodium and calcium.
This solution can be discharged after dilution with
water. Also, nitric acid can be recovered and reused in
some cases as with acrylic fiber spin solutions. Consult
with environmental regulatory agencies for guidance on
acceptable disposal practices. Generators of waste containing
this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment,
and waste disposal. |
| | Nitric acid Preparation Products And Raw materials |
| Raw materials | Ammonia-->Oxygen-->Magnesium oxide-->CARBON MONOXIDE-->Sodium nitrate-->Dehydrolyzing agent-->Platinum-->NITRIC OXIDE-->Magnesium nitrate-->Nitrogen Tetroxide-->Compressor-->Air blower-->Heat exchanger-->cellular membranes | | Preparation Products | 3-METHYLPYRAZINE-2-CARBOXYLIC ACID-->2-Hydroxy-5-nitronicotinic acid-->5-NITROBARBITURIC ACID-->Diethyl nitromalonate-->10-Nitroanthrone-->Lanthanum(III) nitrate hexahydrate-->5-Amino-2,4,6-triiodo-N-methylisophthalamic Acid-->LEAD (II) BORATE MONOHYDRATE-->Cadmium nitrate-->Nessler's Reagent-->4-Acetamido-3-nitrobenzoic acid-->2,6-Dimethyl-3-nitropyridine-->FLAVIANIC ACID-->6-NITROPIPERONAL-->3,5-DINITRO-4-HYDROXYBENZALDEHYDE-->PYRIDINE-2-SULFONIC ACID-->1,3-Dimethyl-8-nitro-1H-purine-2,6(3H,9H)-dione ,97%-->2-NITROTHIOPHENE-4-CARBOXALDEHYDE-->3,3'-DINITROBENZOPHENONE-->2-ethyl-5-nitrobenzenamine-->Mercury iodide-->mercurous bromide-->MERCUROUS CHLORIDE-->PHENYLMERCURY NITRATE-->3-Nitrobenzonitrile-->5-Methylisoxazole-3-carboxylic acid-->2-Nitrothiophene-->2-NITROMESITYLENE-->Sodium nitrohumate-->4,5-DIPHENYLIMIDAZOLE-->2,4-Dichloro-5-nitrobenzalehyde-->5-Chloro-2-hydroxy-3-nitropyridine-->CADMIUM NITRATE TETRAHYDRATE-->hydrofining catalysts (RN series)-->Mesaconic acid-->Methyl 3-nitrobenzoate-->2-HYDROXY-3,5-DINITROPYRIDINE-->MERCUROUS NITRATE-->2-METHYL-5-NITRO-PYRIMIDINE-4,6-DIOL-->TETRANITROMETHANE |
|