- 1-Benzyl-4-piperidone
-

- $60.00 / 1kg
-
2024-05-07
- CAS:3612-20-2
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 2000kg
- N-Benzyl-4-piperidone
-

- $3.00 / 1kg
-
2024-04-12
- CAS:3612-20-2
- Min. Order: 1kg
- Purity: 99.9%
- Supply Ability: 10 tons
|
| | N-Benzyl-4-piperidone Basic information |
| | N-Benzyl-4-piperidone Chemical Properties |
| Boiling point | 134 °C7 mm Hg(lit.) | | density | 1.021 g/mL at 25 °C(lit.) | | vapor pressure | 0.079-0.15Pa at 20-25℃ | | refractive index | n20/D 1.541(lit.) | | Fp | >230 °F | | storage temp. | 2-8°C | | solubility | 12g/l | | form | Oily Liquid | | pka | 7.07±0.20(Predicted) | | Specific Gravity | 1.021 | | color | Clear colorless to straw | | Water Solubility | 12 g/L (20 ºC) | | BRN | 128556 | | LogP | 2.1 at 20℃ and pH9 | | CAS DataBase Reference | 3612-20-2(CAS DataBase Reference) | | NIST Chemistry Reference | 4-Piperidinone, 1-(phenylmethyl)-(3612-20-2) |
| | N-Benzyl-4-piperidone Usage And Synthesis |
| Chemical Properties | Clear colorless to straw coloured oily liquid | | Uses | 1-Benzyl-4-piperidone is a pharmaceutical intermediate, it is used in penfluridol synthesis of bulk drugs. | | Uses | A substituted piperidine as pesticide | | Reactions | As an important organic compound, N-Benzyl-4-piperidone could be used to synthesize many organic compounds. There are four ways to synthesize 1-benzylpiperidine-4-carbaldehyde using it as the raw material.
The first method involves the Wittig reaction of (methoxymethyl) triphenylphosphonium chloride with N-benzyl-4-piperidone, followed by hydrolysis of the resulting enol ether.
In another method, trimethylsilyl diazomethane is condensed with N-benzyl-4-piperidone, followed by hydrolysis to obtain the final product.
In a third route, N-benzyl-4-piperidone is treated with trimethyloxosulfonium iodide to produce the corresponding epoxide. The epoxide is then rearranged in the presence of magnesium bromide etherate, resulting in 1-benzylpiperidine-4-carbaldehyde with high yields.
In 2007, a patent described the Darzens condensation of N-benzyl-4-piperidone with ethyl chloroacetate, followed by decarboxylation[1].
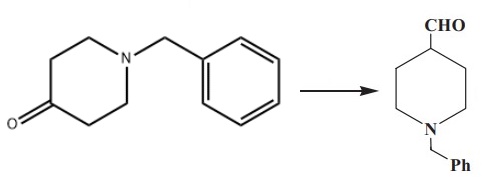 | | Purification Methods | If the physical properties show contamination, then dissolve it in the minimum volume of H2O, made strongly alkaline with aqueous KOH, extract it with toluene several times, dry the extract with K2CO3, filter, evaporate and distil the residue at high vacuum using a bath temperature of 160-190o, and redistil it. [Brookes & Walker J Chem Soc 3173 1957, Bolyard J Am Chem Soc 52 1030 1930.] The hydrochloride has m 159-161o (from Me2CO/Et2O), and the picrate has m 174-182o (from Me2CO/Et2O). [Grob & Brenneisen Helv Chim Acta 41 1184 1958, Beilstein 21/6 V 424.] | | References | [1] Chavakula R, et al. Industrially Viable Preparation ofN-Benzyl-4-formylpiperidine, a Key Intermediate of Donepezil. Organic Preparations and Procedures International, 2013; 45: 168-170. |
| | N-Benzyl-4-piperidone Preparation Products And Raw materials |
| Raw materials | Benzylamine-->Methyl propionate-->4,4-Piperidinediol hydrochloride | | Preparation Products | 4,5,6,7-TETRAHYDRO-2-METHYLFURO[3,2-C]PYRIDINE-->1-BENZYL-4-HYDROXY-4-(3-TRIFLUOROTOLYL)PIPERIDINOL-->1H-Azepine-4-carboxylicacid,hexahydro-,methylester,(4R)-(9CI)-->1H-Azepine-4-carboxylicacid,hexahydro-,methylester,(4S)-(9CI)-->4-(1-Pyrrolidinyl)piperidine-->1-Benzyl-4-hydroxypiperidine-->(1-BENZYLPIPERIDIN-4-YLIDENE)ACETIC ACID ETHYL ESTER-->1-BOC-4-HYDROXY-4-(HYDROXYMETHYL)-PIPERIDINE-->4-(3-Trifuoromethyl)phenyl-4-piperidinol-->N-Boc-2-piperidone-->2-Oxa-8-azaspiro[4.5]decane-->Methyl 1-benzyl-4-hydroxypiperidine-4-carboxylate-->4-(2-Aminoethyl)-1-benzylpiperidine-->4-(4-FLUORO-PHENYL)-PIPERIDIN-4-OL HYDROCHLORIDE-->4-METHYLENEPIPERIDINE-->TERT-BUTYL 1-OXA-4,9-DIAZASPIRO[5.5]UNDECANE-4-CARBOXYLATE |
|