- Drospirenone
-

- $0.00 / 1KG
-
2024-05-07
- CAS:67392-87-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000
- Drospirenone
-

- $0.00 / 1g
-
2024-04-07
- CAS:67392-87-4
- Min. Order: 1g
- Purity: 99.99%
- Supply Ability: 20 tons
- Drospirenone
-

- $0.00 / 1KG
-
2024-03-16
- CAS:67392-87-4
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg
|
| Product Name: | Drospirenone | | Synonyms: | 7β-(HydroxyMethyl)-15β,16β-Methylene-17α-pregn-3,5(6)-diene-21,17-carbolactone;Drospirenone(Dihydrospirorenone);Spiro[17H-dicyclopropa[6,7:15,16]cyclopenta[a]phenanthrene-17,2'(5'H)-furan]-3,5'(2H)-dione;Drospirenone (USP32);Drospirenone, >=98%;Drosporenone;6b,7b:15b,16b-Dimethylen-3-oxo-17a-pregn-4-ene-21;6β,7β:15β,16β -Dimethylene-3-oxo-17α-pregn-4-ene-21,17-carbolactone | | CAS: | 67392-87-4 | | MF: | C24H30O3 | | MW: | 366.5 | | EINECS: | 266-679-2 | | Product Categories: | Other APIs;REBETOL;Intermediates & Fine Chemicals;Pharmaceuticals;Steroids;Steroid and Hormone;67392-87-4 | | Mol File: | 67392-87-4.mol | 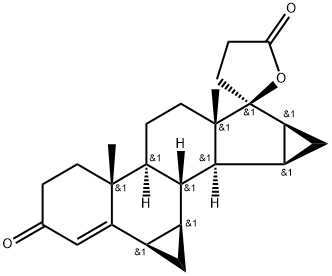 |
| | Drospirenone Chemical Properties |
| Melting point | 196-200°C | | alpha | -180 º (c=0.5, chloroform) | | Boiling point | 552.2±50.0 °C(Predicted) | | density | 1.26±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO: ≥15 | | form | powder | | color | white to tan | | optical activity | [α]/D -180 to -195°, c = 1 in methanol | | InChIKey | METQSPRSQINEEU-URUUTGNFNA-N | | SMILES | C[C@]12CC[C@]3([H])[C@]4(CCC(=O)C=C4[C@@H]4C[C@@H]4[C@@]3([H])[C@]1([H])[C@@H]1C[C@@H]1[C@]12CCC(=O)O1)C |&1:1,4,6,13,15,16,18,20,22,23,r| |
| | Drospirenone Usage And Synthesis |
| Natural progesterone | Drospirenone is the only progesterone that has the characteristics similar to the natural progesterone currently, its structure is more like that of the natural progesterone compare with the structure of the progesterone contained in the traditional oral contraceptives.
Except the effect of anti-androgen, drospirenone has the role of anti-mineralocorticoid which can prevent water and sodium retain in the body. Not only it has the a great effectivenes of contraception, there are also many good impacts on women's health like reducing the acne, making the skin more smooth and controling the weight efficaciously.
Information on the use of oral contraceptives, drospirenone, side effects and other information edited by Chemicalbook maggie. | | Side effects of human estrogen | Drospirenone is a new synthetic progestin designed to mimic the effects of progesterone, it is the preogesterone which the pharmacological properties is closet to that of the natural progesterone, its also has the advantages of both salt corticosteroids and anti-androgen.
As a birth control hormones, Its can reduce the risk of pregnency by imitating progesterone, and increasing the levels of hormones. Therefore drospirenone is promoted as an effective contraception. However, since it has entered the market, drospirenone displays a negative impact on the female estrogen. When two hormones are mixed together, it would bring some bad influence to people.
When drospirenone reacts with estrogen, it can cause the blood thicken. It looks not like a big problem, but the blood clotting would lead to the formation of the thrombosis. The gore forming in the veins can impede blood flow or even plug in some vital organs like the heart, brain and lungs etc when it flowing. The thrombosis would Interference the heart from receiving blood which can cause a heart attack. If it affect the blood flow in brain, it would resulting in a stroke.
There is a possibility of side effects for any women who take the drospirenone, but the people who is obesity, smoking, depression, and have some diseases like the diabetes and high blood pressure are more likely to form the thrombosis when they take the contraception contained the drospirenone. | | Description | Drospirenone is a synthetic progestogen that binds to the progesterone, mineralocorticoid, and androgen receptors with binding affinities of 20, 230, and 65% relative to R5020, aldosterone , and R1881, respectively. In vivo, drospirenone inhibits spontaneous ovulation in rats (ID50s = 0.3-1.0 mg/day) when administered orally or subcutaneously. Drospirenone (0.5 mg/animal) administered six times per day maintains pregnancy in ovariectomized pregnant rats. It reduces serum testosterone (Item Nos. 15645 | ISO60154) and luteinizing hormone in cynomolgus monkeys in a dose-dependent manner. Drospirenone (10 mg/animal per day) also inhibits testosterone-induced growth of the seminal vesicles and prostate in castrated rats. Formulations containing drospirenone have been used as oral contraceptives. | | Description | The steroid drospirenone in combination with the estrogen agonist ethinylestradiol was
introduced in Germany as a new oral contraceptive for women. This analog of the
aldosterone antagonist spironoiactone can be synthesized in five steps from 3β-hydroxy-
15β,16β-methylene-5-androsten-l7-one. Since its binding profile for steroid receptors is
very similar to progesterone, drospirenone mimics the progestogen agonistic activity as
well as the anti-androgenic and anti-mineralocorticoid properties of the endogenous
hormone. In rats, drospirenone inhibited ovulation at 6.3 mglkglday p.o. Compared with
currently available progestins which lack anti-mineralocorticoid activity, drospirenone did
not cause weight gain that could result from fluid retention in clinical studies. Its
combination with ethinylestradiol was well tolerated and did not engender adverse effects
on blood pressure or plasma lipid levels. Drospirenone is rapidly absorbed in man with an
oral bioavailability of 76%. It is extensively metabolized since over 20 different metabolites
were observed in the urine and in the feces, resulting for instance from hydrolysis of the
lactone in the plasma or reductive conjugation of the enone to the 3-sulfate ester of 45
dihydrodrospirenone. Its elimination is bi-exponential with an initial and a terminal half-life
of 2 and 25-33h respectively. | | Chemical Properties | Off-White Crystalline Powder | | Originator | Schering AG (Germany) | | Uses | anti-cancer therapeutic | | Uses | antiviral | | Uses | Synthetic progestogen exhibiting antimineralocorticoid and antiandrogenic activity. | | Uses | Drospirenone has been used as a progestogen agent in pond snail and fish. | | Definition | ChEBI: Drospirenone is a steroid lactone and a 3-oxo-Delta(4) steroid. It has a role as a contraceptive drug, an aldosterone antagonist and a progestin. | | Manufacturing Process | 2.75 g of trimethyl sulfoxonium iodide is stirred in 57 ml of dimethyl sulfoxide
with 341 mg of 80% sodium hydride oil suspension for 2 h at room
temperature. The almost clear solution is combined under nitrogen with 2.0 g
of 15α,16α-methylene-3-oxo-4,6-androstadiene-[17(β-1')-spiro-
5']perhydrofuran-2'-one and agitated for 24 h at room temperature. The
mixture is then stirred into ice water, the thus-obtained precipitate is filtered
off, washed with water, and taken up in methylene chloride. After drying and
evaporation, the residue is purified by repeated preparative layer
chromatography, thus obtaining 520 mg of 6β,7β,15α,16α-dimethylene-3-oxo-
4-androstene-[17(β-1')-spiro-5']perhydrofuran-2'-one (drospirenone). | | Brand name | Yasmin | | Therapeutic Function | Aldosterone antagonist | | General Description | Drospirenone, 3-oxo-6β,7β:15β,16β-dimethylene-17α-pregn-4-en-21,17-carbolactone,differs structurally from all the other commercially availableprogestins. Its structure is similar to that of spironolactone,an MR antagonist, and it does have antimineralocorticoidactivity as well as progestational activity. It isalso reported to have some antiandrogenic effects. Thespirolactone at C17 and the two cyclopropyl groups at C6-C7 and C15-C16 contribute to these unique actions.Drospirenone is the progestin component in the newer oralcontraceptives, Yasmin and Yaz, and in the HRT product,Angeliq. | | Biochem/physiol Actions | Drospirenone is a fourth-generation progestin that has antimineralocorticoid, and antiandrogenic activity in addition to potent progestogenic activity. In two recent studies drospirenone appeared to double the risk of venous thromboembolism compared to levonorgestrel, although other studies found little added risk. |
| | Drospirenone Preparation Products And Raw materials |
|