| Company Name: |
GLP Pharma Standards
|
| Tel: |
+91 9866074638 |
| Email: |
info@glppharmastandards.com |
| Products Intro: |
Product Name:Losartan Azide impurity
CAS:727718-93-6
Purity:98% Package:50 MG; 100 MG; 250 MG ; 1 GM
|
| Company Name: |
ChemStrong Scientific Co.,Ltd
|
| Tel: |
0755-0755-66853366 13670046396 |
| Email: |
sales@chem-strong.com |
| Products Intro: |
Product Name:Losartan Potassium Impurity 1
CAS:727718-93-6
Purity:95% HPLC Package:10mg;25mg;50mg;100mg
|
| Company Name: |
Shanghai Kewel Chemical Co., Ltd.
|
| Tel: |
021-64609169 18901607656 |
| Email: |
greensnown@163.com |
| Products Intro: |
Product Name:Secobarbital-d5 (1-methyl-d3-butyl-2,2-d2)
CAS:727718-93-6
Purity:95+ HPLC; Package:10mg;25mg;50mg;100mg
|
|
| | Losartan Impurity 12 Basic information |
| Product Name: | Losartan Impurity 12 | | Synonyms: | Losartan Impurity 12;Losartan Impurity 21;Losartan Impurity 24;Losartan Impurity 29;Losartan Potassium Impurity 1;Losartan Azide impurityQ: What is
Losartan Azide impurity Q: What is the CAS Number of
Losartan Azide impurity Q: What is the storage condition of
Losartan Azide impurity;Losartan Azide;LosartanImpurity21-d4 | | CAS: | 727718-93-6 | | MF: | C22H22ClN9 | | MW: | 447.93 | | EINECS: | | | Product Categories: | | | Mol File: | 727718-93-6.mol | 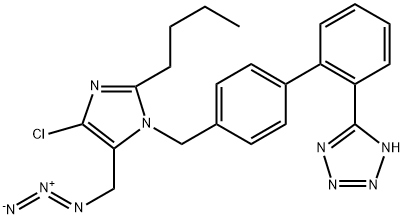 |
| | Losartan Impurity 12 Chemical Properties |
| Melting point | >139°C (dec.) | | storage temp. | -20°C Freezer | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | color | White to Off-White |
| | Losartan Impurity 12 Usage And Synthesis |
| Description | Losartan is a medication used to treat high blood pressure. It is also used for diabetic kidney disease, heart failure, and left ventricular enlargement.Losartan was patented in 1986, and approved for medical use in the United States in 1995. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication.
Losartan was the first nonpeptide imidazole to beintroduced as an orally active angiotensin II antagonist withhigh specificity for AT1. When administered to patients, itundergoes extensive first-pass metabolism, with the 5-methanol being oxidized to a carboxylic acid. This metabolismis mediated by CYP 2C9 and 3A4 isozymes. The 5-methanol metabolite is approximately 15 times more potentthan the parent hydroxyl compound. Because the parent hydroxylcompound has affinity for the AT1 receptor, strictlyspeaking, it is not a prodrug. | | Uses | Losartan Azide is an impurity of Losartan (L470470). Losartan is a nonpeptide angiotensin II AT1-receptor antagonist. Antihypertensive. |
| | Losartan Impurity 12 Preparation Products And Raw materials |
|