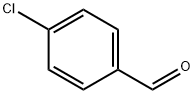
Preparation method and application of 4-chlorobenzaldehyde
Apr 22,2022
Background and overview
4-Chlorobenzaldehyde, also known as p-chlorobenzaldehyde, is colorless flake crystal to light yellow powder. Insoluble in water, soluble in ethanol, ether, benzene, soluble in water and acetone. It can be volatile with water vapor. It is an important intermediate of new pesticides, medicines and dyes. In medicine, p-chlorobenzaldehyde can be condensed and cyclized with mercaptopropionic acid to obtain fennalus. In terms of pesticides, p-chlorobenzaldehyde is used to synthesize herbicides Maidisan, plant growth regulators paclobutrazol and uniconazole, etc. In terms of dyes, p-chlorobenzaldehyde is used to synthesize acid blue 7BF, acid brilliant blue 68, etc.
Preparation
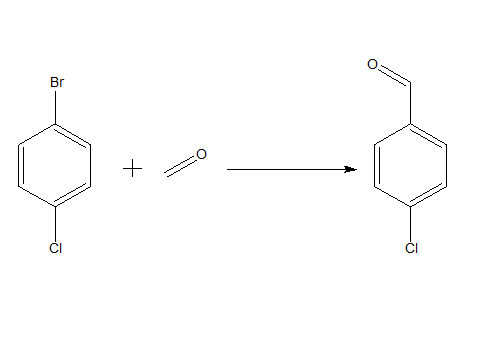
A 50 mL round bottom flask equipped with an inlet tube, reflux condenser, and magnetic stir bar was charged with MCM-41-2PPdCl2 (102 mg, 0.05 mmol Pd), aryl halide (5.0 mmol) and HCOONa (7.5 mmol) ). Rinse the flask with CO. Add DMF (5 mL) via syringe and flow CO slowly into the suspension. The mixture was vigorously stirred at 110-130 °C for 2-20 hours, cooled to room temperature and washed with diethyl ether (50 mL). The palladium catalyst was isolated from the mixture by filtration, washed with distilled water (2 x 10 mL), ethanol (2 x 10 mL) and diethyl ether (2 x 10 mL), and reused in the next run. The ether solution was washed with water (3 x 20 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel flash column chromatography (hexane-ethyl acetate=10:1).
Use
Can be used to prepare 3-(4-chloro-benzylidene)-pentane-2,4-dione
A mixture of aromatic aldehyde (1 mmol), acetylacetone (6 mmol) was charged into a 25 ml round bottom flask equipped with a magnetic stirrer, then PS/TiCl4 (0.12 g, 0.1 mmol TiCl4) containing a certain amount of TiCl4 was added . The resulting mixture was heated to 60°C in a water bath without solvent and stirred for an appropriate period of time. The progress of the reaction was indicated by TLC (silica gel, petroleum ether or hexane/ethyl acetate). After the reaction was completed, the reaction mixture was cooled to room temperature, the catalyst was filtered and washed several times with ethyl acetate. The recovered catalyst was dried at 60°C for 2h. The washings were collected and combined with the filtrate. The organic solution was concentrated/evaporated under reduced pressure in a rotary evaporator, or treated by silica gel column chromatography (elution with a mixture of petroleum ether and ethyl acetate) to obtain a substituted olefin product of satisfactory purity. Data and 1H NMR and IR techniques. The product was characterized by comparing its FT-IR, 1H NMR and physical data with that of the real sample. Conversion is defined as the percentage of starting aldehyde converted to product. Characterize products by comparing their physical data with those of known samples or by comparing their IR and NMR.
Can be used to prepare (4-chloro-benzylidene)-methylamine
A 33% solution of anhydrous ethanol in methylamine (0.283 g, 0.375 mL, 3.0 mmol), 4-chlorobenzaldehyde (2.0 mmol) and TMDP (0.087 g, 0.4 mmol) were successively added to the reaction flask and heated at 85 °C Stir under vigorous heating for 90 minutes. After completion of the reaction (monitored by TLC), deionized water (2 mL) was added to the reaction mixture, and the crude product was extracted with ethyl acetate (5 x 2 mL). Aqueous TMDP and residual methylamine were separated while the combined ethyl acetate extracts were concentrated in vacuo and detected by GC-MS. No signal for TMDP was detected, confirming that the extraction procedure did not extract TMDP. The crude product was dissolved by heating with absolute ethanol, then cooled and crystallized, and the purified product was obtained after recrystallization. The melting point and 1H NMR spectrum of the obtained compound are consistent with those reported in the literature.
Used in organic synthesis to prepare other pharmaceutical or fine chemical intermediates.
References
【1】Journal of Chemical Research,2014, vol. 38, # 4, p. 218 - 222
【2】Catalysis Communications,2019, vol. 124, p. 24 – 31
【3】Research on Chemical Intermediates,2022
- Related articles
- Related Qustion
4-Chlorobenzaldehyde
104-88-1You may like
- What is the crystal structure of nickel silicide?
May 21, 2024
- What is the crystal structure of chromium silicide?
May 21, 2024
- Crystal Structure of Tantalum nitride
May 21, 2024
4-Chlorobenzaldehyde manufacturers
- 4-Chlorobenzaldehyde
-

- $1.00 / 1g
- 2024-05-21
- CAS:104-88-1
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- 4-Chlorobenzaldehyde
-

- $5.00 / 200KG
- 2024-05-21
- CAS:104-88-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500mt/year
- 4-Chlorobenzaldehyde
-

- $0.00 / 1KG
- 2024-03-16
- CAS:104-88-1
- Min. Order: 100g
- Purity: 98%+
- Supply Ability: 100kg