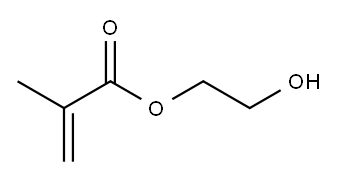
Is 2-hydroxyethyl methacrylate safe?
May 7,2024
HEMA (2-hydroxyethyl methacrylate), commonly used in dentistry, was reported to induce genotoxic effects. Several papers addressed the genotoxicity of methacrylates used in dental practice, mainly in vitro. It follows from those reports that methacrylates may induce DNA damage, mutations, apoptosis, cell cycle perturbation, and gene expression change. However, a direct interaction between methacrylates and DNA was shown in very few studies. Methacrylate monomers, including HEMA, could interact with the DNA of human lymphocytes, inducing single and double-strand breaks and alterations to the DNA bases, including oxidative modifications. These effects were not associated with significant changes in cell viability but were linked with apoptosis and cell cycle changes.

In searching for the mechanism underlying the genotoxic effects of 2-Hydroxyethyl methacrylate, one should consider the degrading action of the oral cavity esterases. A proposed degradation pathway of HEMA suggests that its most potent genotoxic intermediate is 2,3-epoxy-methacrylic acid. In fact, epoxy compounds were reported to display genotoxic properties. However, the genotoxic action of MAA cannot be excluded, especially since this compound was reported to stimulate the release of tumor necrosis factor β and interleukin-6 in mouse macrophages. Therefore, the assessment of the genotoxic properties of MAA in comparison with its parent compound, HEMA, is justified. Researchers compared the genotoxic effects of HEMA and MAA in human gingival fibroblast. They investigated the ability of both compounds to induce DNA damage, apoptosis, and cell cycle perturbations.
2-Hydroxyethyl methacrylate may be degraded by the oral cavity esterases or through mechanical stress following the chewing process. Methacrylic acid (MAA) is the primary product of HEMA degradation. A 6-h exposure to HEMA or MAA induced a weak decrease in the viability of HGFs. Neither HEMA nor MAA induced strand breaks in the isolated plasmid DNA, but both compounds evoked DNA damage in HGFs, as evaluated by the alkaline comet assay. Oxidative modifications to the DNA bases were monitored by the DNA repair enzymes Endo III and Fpg. DNA damage induced by HEMA and MAA was not persistent and was removed during a 120-minute repair incubation. Results from the neutral comet assay indicated that both compounds induced DNA double-strand breaks (DSBs), and they were confirmed by the γ-H2AX assay. Both compounds induced apoptosis and perturbed the cell cycle. Therefore, methacrylic acid, a product of HEMA degradation, may be involved in its cytotoxic and genotoxic action.
Reference
[1] Joanna Szczepanska. “2-hydroxylethyl methacrylate (HEMA), a tooth restoration component, exerts its genotoxic effects in human gingival fibroblasts trough methacrylic acid, an immediate product of its degradation.” Molecular Biology Reports 39 2 (2012): 1561–74.
- Related articles
- Related Qustion
- What is 2-Hydroxyethyl Methacrylate? Jan 13, 2020
2-hydroxyethyl methacrylate (HEMA) is a commercially important and widely used monomer. It is commonly prepared in a one-step reaction from methyl methacrylate or methacrylic acid.
Tert-butyl bromoacetate is crucial in synthesizing high-performance MRI contrast agents, enhancing imaging quality, while its green preparation promotes sustainability and efficiency.....
May 7,2024API(2-Bromoethyl)benzene is essential in pharmaceutical synthesis, enabling the creation of potent antimicrobial compounds, and is synthesized through specific reactions.....
May 7,2024API2-Hydroxyethyl methacrylate
868-77-9You may like
- The toxicity of Triethylene glycol
May 14, 2024
- Is 1,4-benzoquinone a toxicity compound?
May 11, 2024
- The Synthesis method and Toxicity of 18-Crown-6
May 10, 2024
2-Hydroxyethyl methacrylate manufacturers
- 2-Hydroxyethyl methacrylate (HEMA)
-

- $0.00 / 200Kg/Drum
- 2024-05-29
- CAS:868-77-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000mt/year
- 2-Hydroxyethyl methacrylate
-

- $2375.00 / 1Tons
- 2024-05-29
- CAS:868-77-9
- Min. Order: 1Tons
- Purity: 99%
- Supply Ability: 100Tons
- 2-Hydroxyethyl methacrylate,HEMA
-

- $0.00 / 200KG
- 2024-05-27
- CAS:868-77-9
- Min. Order: 200KG
- Purity: 98
- Supply Ability: 10mt