Tert-Butyl Bromoacetate: Applications in the Synthesis of MRI Contrast Agents and Green Preparation
May 7,2024
General Description
The synthesis of MRI contrast agents utilizing tert-butyl bromoacetate demonstrates its crucial role in advancing medical imaging technology. Tert-butyl bromoacetate contributes to the development of high-performance contrast agents like [Gd(AAZTA-4H)]- and [Fe{Gd2L(H2O)4}3]4-, which exhibit exceptional magnetic properties and proton relaxivity, enhancing the quality of MRI imaging. Tert-butyl bromoacetate's involvement underscores its significance in improving diagnostic capabilities and accuracy in MRI procedures. Furthermore, the green preparation method for tert-butyl bromoacetate emphasizes sustainability and efficiency in its synthesis, showcasing a commitment to environmental friendliness. This method ensures high purity, cost-effectiveness, reduced environmental impact, and enhanced safety measures, aligning with the principles of green chemistry. Overall, tert-butyl bromoacetate plays a pivotal role in the development of cutting-edge MRI contrast agents, highlighting its importance in the field of medical imaging and its potential to contribute to the creation of more effective and reliable contrast agents for MRI applications.

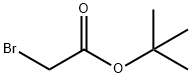
Figure 1. tert-Butyl bromoacetate
Applications in the Synthesis of MRI Contrast Agents
Tert-butyl bromoacetate, a versatile compound, finds application in the synthesis of MRI contrast agents, contributing to advancements in medical imaging technology. One such innovative contrast agent is based on the ligand AAZTA, which can be easily synthesized. The corresponding Gd(III) chelate, [Gd(AAZTA-4H)]-, exhibits exceptional magnetic properties and high thermodynamic stability in aqueous solutions. Additionally, it demonstrates remarkable inertness towards bidentate endogenous anions, positioning it as a highly promising candidate for the development of high-performance MRI contrast agents. The unique properties of this compound make it an attractive option for enhancing the quality of MRI imaging. Furthermore, a metallostar complex, [Fe{Gd2L(H2O)4}3]4-, also showcases significant potential as an MRI contrast agent despite its moderate molecular weight. This complex displays exceptionally high proton relaxivity, attributed to its rigid supramolecular structure, optimal exchange rate of inner-sphere water molecules, and the presence of six efficiently relaxing GdII centers within a confined molecular space. These features contribute to its ability to provide high-quality imaging results despite its relatively small molecular size. The utilization of tert-butyl bromoacetate in the synthesis of these advanced MRI contrast agents underscores its importance in the development of cutting-edge medical imaging technologies. By contributing to the creation of contrast agents with enhanced magnetic properties, stability, and imaging performance, tert-butyl bromoacetate plays a crucial role in improving the accuracy and diagnostic capabilities of MRI procedures. This application highlights the compound's significance in the field of medical imaging and its potential to contribute to the development of more effective and reliable contrast agents for MRI. 1
Green Preparation
The green preparation method for tert-butyl bromoacetate is a sustainable and efficient process that prioritizes environmental friendliness. This synthesis occurs in tetrahydrofuran solvent at a controlled temperature of 10-15°C, utilizing solid super-strong acid as a catalyst under normal pressure conditions. The reaction mechanism involves a low-temperature acid esterification process, ensuring high efficiency and minimal waste generation. Following the reaction, the catalyst is carefully filtered and recovered for reuse, showcasing a commitment to resource efficiency. The filtrate undergoes solvent recovery, and through a distillation process, tert-butyl bromoacetate is obtained with a purity exceeding 99.0%. Key aspects of this green synthesis method include the use of solid perfluorosulfonic acid resin as the catalyst, precise temperature regulation (around 10°C) during the 5-hour reaction time, a specific molar ratio (1:1.1-1.5) for bromoacetic acid and isobutene, and a defined catalyst to bromoacetic acid mass ratio (1:8-10). This method not only ensures a high level of purity but also offers advantages such as cost-effectiveness, simplicity in operation, reduced environmental impact, and enhanced safety measures. By adhering to the principles of green chemistry, this approach is well-suited for industrial-scale production of tert-butyl bromoacetate while promoting sustainability and eco-friendliness in chemical synthesis processes. 2
Reference
1. Livramento JB, Tóth E, Sour A, Borel A, Merbach AE, Ruloff R. High relaxivity confined to a small molecular space: a metallostar-based, potential MRI contrast agent. Angew Chem Int Ed Engl. 2005; 44(10): 1480-1484.
2. Zhou YL. Process for environment-friendly synthesis of tert-butyl bromoacetate. 2017; Patent Number: CN106380398.
- Related articles
- Related Qustion
- Tert-Butyl bromoacetate: applications as alkylating agent and safety Dec 15, 2023
Tert-butyl bromoacetate is a versatile alkylating agent in synthetic processes, enhancing compound properties but requiring careful handling due to significant health hazards.
- tert-Butyl bromoacetate: Background technique, synthesis, applications and hazard May 25, 2023
tert-Buty bromoacetate is used for the synthesis of amino acids and peptides, and the synthesis of cyclic polyamino carboxylate contrast agents, cephalosporins, and Receptor antagonists
- About tert-Butyl bromoacetate Aug 19, 2019
The invention relates to a synthesis method of tert-butyl bromoacetate, and the synthesis method comprises the following steps of: mixing bromoacetic acid and tert-butyl acetate at a weight ratio of 4:(1-1):1, and completely dissolving bromoacetic acid in tert-butyl acetate; then, feeding the solution into a reactor filled with strong acid type ion exchange resin, controlling the temperature within the range of 30-45 DEG C,
4-Bromoaniline is a versatile compound used in various applications, with its metabolism in rats studied and its synthesis involving amination of Bromobenzene yielding 100% product.....
May 7,2024APIHEMA (2-hydroxyethyl methacrylate), commonly used in dentistry, was reported to induce genotoxic effects.....
May 7,2024Organic reagentstert-Butyl bromoacetate
5292-43-3You may like
tert-Butyl bromoacetate manufacturers
- tert-Butyl bromoacetate
-

- $5.00 / 250KG
- 2024-05-24
- CAS:5292-43-3
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 500mt/year
- tert-Butyl bromoacetate
-

- $0.00 / 1kg
- 2023-11-15
- CAS:5292-43-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000kg
- tert-Butyl bromoacetate
-

- $0.00 / 1kg
- 2023-10-14
- CAS:5292-43-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20tons