OMadacycline: Mechanism, Bioactivity and Chemical and Toxicological Studies
Jan 3,2023
General description
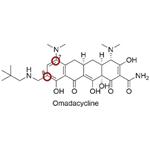
OMadacycline, with the CAS No:1075240-43-5, is also known as (4S, 4aS,5aR,12aS) -4,7-bis(dimethylamino)-9-(2,2-dimethylpropylaminomethyl)-3,10,12,12a-tetra-hydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetra-cene-2-carboxamide4-methylbenzene sulfonate. This chemical’s molecular formula is C36H48N4O10S and molecular weight is 728. 9. It is an aminomethylcycline tetracycline antibiotic for the treatment of community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI).[1]
OMadacycline discovered by Paratek, is a novel and modernized semi-synthetic antibiotic in the tetracycline class known as the aminomethylcyclines containing a neopentylaminomethyl group attached at the C9 position. OMadacycline represents a significant advance within this class of antibiotics due to its activity against tetracycline resistant organisms with either ribosomal protection and/or efflux mechanism of resistance. In vitro studies have shown it to have activity against the vast majority of gram-positive pathogens, including multi-drug resistant Staphylococcus aureus and vancomycin-resistant Enterococci. It has also shown improved activity over minocycline against a wide spectrum of gram-negative and anaerobic clinical bacterial isolates. It exhibits activity across a spectrum of bacteria, including Gram-positive, Gram-negative, atypicals, and other drug-resistant strains.Both oral and intravenous formulations were developed for clinical testing.[2] Omadacycline, the drug that US FDA approved for the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. It is not susceptible to common tetracycline-resistancemechanisms, and has demonstrated efficacy against a broad spectrum of pathogens including resistant isolates, which are increasing in prevalence and complexity. It is available in both intravenous and oral formats, and can be administered in single, once daily doses or multiple doses, with no dosing adjustments required for sex, age, hepatic or renal impairment. It can be a good option for patients with low treatment adherence, and oral therapy may be used to reduce length of hospitalization for iv. treatment.[4]
Figure 1 the molecular formula of Omadacycline
Pharmacodynamics
Omadacycline has shown clinical efficacy in anaerobic acute bacterial skin and skin structure infections (ABSSSI) and in animal models of intra-abdominal anaerobic infections. The in vitro activity of Omadacycline against clinically relevant anaerobes was similar to that of tigecycline, with MIC90 values of 1 to 8 g/ml against Bacteroides spp, 0.5 g/ml against Clostridium difficile, Prevotella spp., and Porphyromonas asaccharolytica,1 g/ml against Peptostreptococcus spp, and 16 g/ml against Clostridium perfringens.[3] It has excellent activity against pathogens from the respiratory tract and overcomes tetracycline resistance. And It was very active against SPN, regardless of PEN-S status, with MIC50/90 value of 0.06/0.06 mg/L and no MIC value greater than 0.25 mg/L. PTK 0796 was also very active against M. catarrhalis (MIC50/90, 0.12/0.12 mg/L) and H. influenzae (MIC50/90, 0.5/1 mg/L) with activity independent of beta-lactamase status.[5] An oral-only omadacycline regimen was non-inferior to oral linezolid for the treatment of adults with ABSSSI. Omadacycline had a similar safety profile to linezolid. Considering the substantial burdens associated with initial inpatient management of skin infections, oral omadacycline is a new once-daily option to treat ABSSSI that might be considered as an alternative to linezolid.[6]
Omadacycline had potent activity in vitro against Gram-negative and -positive anaerobes commonly isolated from human infections. The activity of Omadacycline against anaerobes was similar to that reported previously and also parallels that observed with TGC, an agent indicated for the treatment of anaerobes in skin and intra-abdominal infections, both by MIC50/90 and MIC distribution, with values identical or within 2-fold. The in vitro activity of Omadacycline against anaerobic pathogens along with the in vivo efficacy against anaerobes in animal models of anaerobic infection and in human skin infections highlight the potential of Omadacycline for the treatment of human anaerobic infections.[3]
Omadacycline can also acute bacterial skin and skin structure infections and community-acquired pneumonia as both an oral and intravenous, once-daily formulation. In this report omadacycline and comparators were tested against 69,246 non-duplicate bacterial isolates collected prospectively during 2010 and 2011 from medical centers in Asia-Pacific (11,397 isolates), Europe (23,490 isolates), Latin America (8,038 isolates) and North America (26,321 isolates). Omadacycline was tested by broth microdilution following Clinical and Laboratory Standards Institute M07-A10 (2015) methods. A total of 99.9% of Staphylococcus aureusisolates were inhibited by ≤2 µg/ml of omadacycline (MIC50/90, 0.12/0.25 µg/ml) including 100.0% of methicillin-resistant S. aureus and 99.8% of methicillin-susceptible S. aureus. Omadacycline potency was comparable for Streptococcus pneumoniae (MIC50/90, 0.06/0.06 μg/ml), viridans group streptococci (MIC50/90, 0.06/0.12 μg/ml) and β- hemolytic streptococci (MIC50/90, 0.06/0.12 μg/ml) regardless of species and susceptibility to penicillin. Omadacycline was active against Enterobacteriaceae and was most active against Escherichia coli (MIC50/90, 0.5/2 µg/ml), E. aerogenes (MIC50/90, 2/4 µg/ml), Klebsiella oxytoca (MIC50/90, 1/4 µg/ml) and Citrobacter spp. (MIC50/90, 1/4 µg/ml). Omadacycline was active against Haemophilus influenzae (MIC50/90, 1/1 μg/ml) regardless of β-lactamase status and against Moraxella catarrhalis (MIC50/90, 0.12/0.25 μg/ml). The potent activity of omadacycline against Gram-positive and negative bacteria indicates that omadacycline merits further study in serious infections in which multidrug resistance and mixed Gram-positive and -negative infections may be a concern.[7]
Omadacycline (MIC50/90 , 0.12/0.25 mg/L) was highly active against S. aureus isolates from skin and skin structure infection (SSSI; 99.3% susceptible [S]) including MRSA (97.7%S) and MSSA (99.9%S). Similarly, Omadacycline demonstrated potent activity against S. aureus isolates from respiratory tract infection (RTI; MIC50/90 , 0.12/0.25 mg/L) including MSSA (98.2%S). All S. lugdunensis isolates from SSSI were S (100.0%) to OMC. All Streptococcus anginosus group (100.0%) and 97.6% of S. pyogenes isolates from SSSI were S to Omadacycline as were 98.0% of S. pneumoniae from RTI. No streptococci were resistant (R) to Omadacycline. Omadacycline (MIC50/90 0.12/0.25 mg/L) had potent activity against E. faecalis isolates from SSSI (99.0%S). Omadacycline S against E. cloacae and K. pneumoniae isolates from SSSI was 92.1% S and 89.4% S, respectively. Similarly, 86.2% of K. pneumoniae isolates from RTI were S to Omadacycline. Susceptibility of H. influenzae isolates from RTI to Omadacycline was 99.8%S (no isolates were R). ≥90.0% of E. coli (MIC50/90 , 1/2 mg/L) and K. pneumoniae (MIC50/90 , 2/4 mg/L) UTI isolates were inhibited by ≤4 mg/L of Omadacycline. And Omadacycline was highly active against bacterial pathogens associated with ABSSSI, CABP, and UTI including staphylococci (97.7%-100.0%S), streptococci (97.6%-100.0%S), E. faecalis (99.0%S). E. cloacae (92.1%S), K. pneumoniae (86.2%89.4%S), and E. coli. [8] In Phase 1 studies asymptomatic increases in heart rate (HR) were observed following OMC dosing in healthy volunteers. In vitro studies showed Omadacycline inhibits binding of acetylcholine to the M2 -subtype of the muscarinic receptor, resulting in a nonadrenergic, vagolytic effect. In a thorough QT study, Omadacycline had no clinically meaningful effect on the QTc interval or any other ECG parameters. Phase 3 studies of Omadacycline as an oral (PO) and intravenous (IV) monotherapy for ABSSSI and CABP were recently completed.[9] Phase 2 Omadacycline clinical trials for uncomplicated urinary tract infection (uUTI; NCT03425396) and acute pyelonephritis (NCT03757234) are ongoing. Omadacycline is active against bacterial isolates expressing common tetracycline, penicillin, fluoroquinolone, and macrolide resistance mechanisms.[8]
Mechanism of action
Omadacycline is from minocycline that circumvents the efflux and ribosomal protection mechanisms of tetracycline-specific resistance, restoring activity in vitro and in vivo against common community-acquired skin pathogens, including staphylococci (eg, meticillinsusceptible S aureus [MSSA] and MRSA), streptococci, Enterococcus species, and many Gram-negative bacilli.[6]
Preparation
In step 1, minocycline hydrochloride was reacted with nearly three equivalents of hydroxymethylphthalimide in triflic acid. Compound 1 possesses several reactive function groups, the C1 primary amide being more reactive than C9 or the C10 phenol. Due to this fact, the reaction afforded a mixture of bis- and tris-adducts 3 and 4. In step 2, the phthalimides were deprotected with large excess ofmethylamine to afford a mixture ofmono- and bis-aminomethyl 5 and 6, along with solid waste phthalamide 7. Compound 5 was found to be unstable and required storage at sub-ambient temperatures. In step 3, de-aminomethylation of the primary amide and a concomitant reductive amination of C9 methylamine with pivaldehyde afforded crude OMadacycline. After reverse phase chromatographic purification, pH adjustment and precipitation afforded OMadacycline as an amorphous, unstable solid. The overall crude yield of OMadacycline was about 35%, and 50% was recovered after the purification.[2]

Figure 2 Preparation of Omadacycline
Toxicity
OMadacycline may cause serious adverse reactions including hypersensitivity reactions, tooth discoloration, Clostridioides difficile-associated diarrhea, and reversible inhibition of bone growth when administered during the second and third trimesters of pregnancy. Common adverse reactions include nausea, vomiting, infusion site reactions, alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyl transferase increased, hypertension, headache, diarrhea, insomnia, and constipation.[1]Mild to moderate nausea and vomiting were the most frequent treatment-emergent adverse events in omadacycline (111 [30%] of 368 and 62 [17%] of 368, respectively) groups.[6]
References
[1]Wang M J, Dong J H. Omadacycline tosylate (Nuzyra)[J]. Chinese Journal of Medicinal Chemistry, 2019(4):1.
[2]Chung J, Hartner F W, Cvetovich R J. Synthesis development of an aminomethylcycline antibiotic via an electronically tuned acyliminium Friedel–Crafts reaction[J]. Tetrahedron Letters, 2010, 40(4):6095-6100.
[3]Stapert L, Wolfe C, Shinabarger D, et al. In Vitro Activities of Omadacycline and Comparators against Anaerobic Bacteria[J]. Antimicrobial agents and chemotherapy. 2018(4):62.
[4]Chopra T, Sandhu A, Theriault N, et al. Omadacycline: A therapeutic review of use in community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections[J]. Future Microbiology, 2020, 15(14).
[5]Flamm R K, Farrell D J, Sader H S, et al. Antimicrobial Activity of PTK 0796 (Omadacycline) and Comparator Agents Against Contemporary Pathogens Commonly Associated with Community-Acquired.
[6]William O, Carrie C, Elliot S, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial[J]. The Lancet. Infectious diseases, 2019, 19(10):1080-1090.
[7]Pfaller M A, MD Huband, Rhomberg P R, et al. Surveillance of Omadacycline Activity against Clinical Isolates from a Global Collection (North America, Europe, Latin America, Asia-Western Pacific), 2010-2011[J]. Antimicrobial Agents & Chemotherapy, 2017, 61(5).
[8]MD Huband, Pfaller M A, Streit J M, et al. 665. In vitro Activity of Omadacycline Against Recent (2018) Bacterial Pathogens from the United States and Europe Obtained from Skin and Skin Structure, Respiratory, and Urinary Tract Infections[J]. Open Forum Infectious Diseases, 2019.
[9]Borje D, Evan T, Lynne G R, et al. Cardiac Safety of Omadacycline in the IV/oral Phase 3 Acute Bacterial Skin and Skin Structure Infection (ABSSSI) and in the IV/oral Phase 3 Community-acquired Bacterial Pneumonia (CABP) Studies[J]. Open Forum Infectious Diseases, 2017.
- Related articles
- Related Qustion
- OMadacycline (Tosylate): A Promising Tetracycline Antibiotic for the Treatment of Bacterial Infections Jan 29, 2024
OMadacycline (tosylate) is a potent tetracycline antibiotic that inhibits bacterial protein synthesis by binding to ribosome, with broad effectiveness against resistant strains and moderate half-life.
Polyvinyl butyral (PVB) is a well-known thermoplastic (noncross-linked) encapsulant. It has been used for a long time in architecture for safety-glass laminates as well as in the PV industry for building-integrated photovoltaics (BIPV) and....
Dec 30,2022Catalyst and AuxiliaryCollagen is the most abundant protein found in the body. These proteins form a mesh of fibers that help provide support and flexibility to the body’s tissues and form key components that lead to healthy muscle, skin, bones and tendons.....
Jan 3,2023APIOMadacycline (tosylate)
1075240-43-5You may like
OMadacycline (tosylate) manufacturers
- Omadacycline Tosylate
-
- $0.00 / 1g
- 2024-02-06
- CAS:1075240-43-5
- Min. Order: 1g
- Purity: 0.99
- Supply Ability: 100kg







