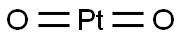Unlocking the Versatility of Platinum Dioxide in Catalyst, Electronics, and Biomedical Applications
Jan 5,2024
General Description
Platinum dioxide, with its unique properties and versatile applications, is a valuable compound in various industries. It exhibits stability, conductivity, and reactivity, making it suitable for catalysis reactions, electronics, and biomedical engineering. Its role as a catalyst in automotive catalytic converters and fuel cells helps reduce environmental pollution and generate electricity. However, safety precautions are essential when handling Platinum dioxide due to its irritating and corrosive nature. Protective equipment, proper ventilation, and avoiding ingestion and inhalation are crucial to minimize health risks. By following these measures, the potential benefits of Platinum dioxide can be harnessed while ensuring safety.

Figure 1. Platinum dioxide
Unique Features
In addition to its limited solubility in water and dilute acids, Platinum dioxide exhibits other unique properties. It is highly stable at room temperature but can react with reducing agents like hydrogen gas or carbon monoxide to form metallic platinum. Platinum dioxide is also a good conductor of electricity and is often used as an electrode material in electrochemical processes. Its high surface area and conductivity make it useful in catalysis reactions, such as in the automotive industry to reduce vehicle emissions. Furthermore, Platinum dioxide has potential applications in the medical field due to its biocompatibility and antimicrobial properties. Research suggests that it could be used in the development of biomedical devices and implants. Overall, Platinum dioxide's distinct properties make it a valuable material for various industries and scientific fields. Its stability, conductivity, and reactivity properties offer numerous applications from electrochemistry to biomedical engineering. 1
Applications in Various Industries
Platinum dioxide is a versatile compound that has found numerous applications in various industries. Its high oxidation state makes it an excellent catalyst for various chemical reactions, and it is used in the preparation of other platinum compounds. In the field of electronics, Platinum dioxide serves as a resistor in specific types of electrical circuits and is an essential component in the production of many electronic devices due to its stable resistance values. The automotive industry uses Platinum dioxide in the manufacturing of catalytic converters, which help reduce harmful gases from vehicle exhausts, playing a vital role in mitigating environmental pollution. The compound also plays a crucial role in fuel cells as a catalyst in the chemical reactions that generate electricity. Nanoparticles of Platinum dioxide are being investigated in the field of nanotechnology for use in biomedical applications such as cancer therapy due to their unique interactions with biological systems. With its diverse range of applications, Platinum dioxide continues to be a sought-after compound in various industries, including automotive, electronics, and energy. 2
Essential Safety Measures
While Platinum dioxide has significant industrial applications, its safety should not be overlooked. It is classified as an irritant and can cause irritation to the eyes and skin. Therefore, it is crucial to handle it with care and use appropriate protective equipment such as gloves and eye protection. Platinum dioxide is also considered a corrosive substance to the eyes and may cause serious eye irritation. It is an oxidizing agent and can lead to fires when in contact with combustible materials. Ingestion and inhalation of Platinum dioxide should be strictly avoided, as it can pose serious health risks. If accidental ingestion or inhalation occurs, immediate medical attention is necessary. To minimize the risk of exposure, it is essential to ensure adequate ventilation or exhaust systems are in place when working with Platinum dioxide. This helps to reduce the concentration of the compound in the air and lowers the chances of inhalation. In summary, while Platinum dioxide has valuable applications, proper safety precautions must be taken when handling it. Protective equipment, such as gloves and eye protection, should be used, and ingestion and inhalation should be avoided. Adequate ventilation should be maintained to minimize the risk of exposure. By following these safety measures, the potential risks associated with Platinum dioxide can be effectively mitigated. 3
Reference
1. Platinum(IV) oxide. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 345198.
2. Platinic oxide. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases, 8669.
3. Platinum dioxide. European Chemicals Agency, 32453.
- Related articles
- Related Qustion
- Platinum Dioxide: Characteristics, Synthesis Method and Safety Apr 25, 2024
Platinum dioxide, valued for stability and conductivity, is synthesized by heating platinum in oxygen. Caution is necessary due to fire and health risks, stressing safety protocols.
- Platinum dioxide:Application and Chemical Studies Feb 14, 2023
Platinum dioxide as a noble metal oxide catalyst, has a wide range of applications in hydrogenation, hydrogenolysis, dehydrogenation and oxidation reactions.
Sodium tungstate has potent antioxidant and antidiabetic effects, can enhance cancer treatment but requires further safety investigation.....
Jan 5,2024APIEthyl vanillin's applications include enhancing food packaging, exhibiting antibacterial properties, and showing potential as an antioxidant, despite potential adverse effects at high doses.....
Jan 5,2024APIPlatinum dioxide
1314-15-4You may like
Platinum dioxide manufacturers
- Platinum dioxide
-

- $30.00 / 1g
- 2024-05-29
- CAS:1314-15-4
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 300tons
- Platinum dioxide
-

- $5.00/ kg
- 2024-04-18
- CAS:1314-15-4
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 10000ton
- Platinum dioxide
-

- $0.00 / 1kg
- 2024-02-29
- CAS:1314-15-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000