- Platinum dioxide
-

- $30.00 / 1g
-
2024-05-28
- CAS:1314-15-4
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 300tons
- Platinum dioxide
-

- $5.00/ kg
-
2024-04-18
- CAS:1314-15-4
- Min. Order: 1kg
- Purity: 99.8%
- Supply Ability: 10000ton
- Platinum dioxide
-

- $0.00 / 1kg
-
2024-02-29
- CAS:1314-15-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000
|
| Product Name: | Platinum dioxide | | Synonyms: | PLATINUM OXIDE (Black);Platinum(IV) oxide, anhydrous, Premion, 99.95% (metals basis), Pt 84.4% min;PlatinuM(Ⅱ)oxide;PlatinuM oxide anhydrous;PlatinuM(IV) oxide, 83% Pt 1GR;PlatinuM(IV) oxide, 83% Pt 5GR;PLATINUM-(IV)-OXIDE, ANHYDROUS;Platinium (IV)oxide anhydrous | | CAS: | 1314-15-4 | | MF: | O2Pt | | MW: | 227.08 | | EINECS: | 215-223-0 | | Product Categories: | chemical reaction,pharm,electronic,materials;Inorganics;1314-15-4 | | Mol File: | 1314-15-4.mol | 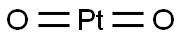 |
| | Platinum dioxide Chemical Properties |
| Melting point | 450 °C (lit.) | | density | 10.2 | | RTECS | TP2506020 | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | form | crystalline | | color | Dark brown | | Water Solubility | Soluble in caustic potash solution. Insoluble in water, acid, aquaregia. | | Merck | 14,7527 | | Stability: | Contact with combustible material may cause fire. Incompatible with organic materials, powdered metals. | | InChI | InChI=1S/2O.Pt | | InChIKey | WNVYCMIJBVIKSY-UHFFFAOYSA-N | | SMILES | [Pt](=O)=O | | CAS DataBase Reference | 1314-15-4(CAS DataBase Reference) | | NIST Chemistry Reference | Platinum dioxide(1314-15-4) | | EPA Substance Registry System | Platinum oxide (PtO2) (1314-15-4) | | solubility | THF, o-xylene, chloroform, chlorobenzene and dichlorobenzene |
| | Platinum dioxide Usage And Synthesis |
| Product Features | Platinum oxide whose chemical formula is PtO2 is used as Adams catalyst in organic synthesis. Its molecular weight is 227.03. It is brown-black powder or black solid; the melting point of it is 450 ℃ and the relative density is 10.2. It doesn’t dissolve in water, concentrated acid and aqua regia. It will be decomposed into oxygen and platinum when heated to 500 ℃. It can be reduced by hydrogen or carbon monoxide. It can be dissolved to generate platinum oxide (Ⅱ) when heated in sulfurous acid. There are a variety of hydrates of platinum oxide, such as dihydrate and trihydrate which is difficult to dissolve in sulfuric acid or nitric acid but soluble in hydrochloric acid or sodium hydroxide solution, and monohydrate insoluble in hydrochloric acid, or even aqua regia. PtO2 powder can be prepared generally from the melting chloroplatinic acid and sodium nitrate at about 500~550 ° C followed by the dissolution of the remaining nitrate in water and filtration. The trihydrate can be obtained when the yellow hexahydroxy platinic acid precipitate is heated black from brown, which is obtained after the boiling and cooling of the mixture of hexachloroplatinic acid and excess 2mol/L sodium hydroxide, followed by the neutralization of excess base. The trihydrate dried in sulfuric acid in a desiccator will generate dihydrate, which is then heated to 100 ° C to produce a monohydrate which is very difficult to dehydrate. Platinum oxide is widely used as a hydrogenation catalyst in organic synthesis (refer to catalytic hydrogenation reaction). However latinum black generated from the hydrogen reduction of platinum dioxide in the reaction acts as the actual catalyst. | | Platinum Oxide | Platinum Oxide, or Platinum Dioxide, is a highly insoluble thermally stable Platinum source suitable for glass, optic and ceramic applications. Platinum oxide is a dark brown powder also known as Adam's Catalyst; it only becomes an active catalyst with exposure to Hydrogen. Oxide compounds are not conductive to electricity. However, certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systems. They are compounds containing at least one oxygen anion and one metallic cation. High Purity (99.999%) Platinum Oxide (PtO2) PowderThey are typically insoluble in aqueous solutions (water) and extremely stable making them useful in ceramic structures as simple as producing clay bowls to advanced electronics and in light weight structural components in aerospace and electrochemical applications such as fuel cells in which they exhibit ionic conductivity. Metal oxide compounds are basic anhydrides and can therefore react with acids and with strong reducing agents in redox reactions. Platinum Oxide is also available in pellets, pieces, powder, sputtering targets, tablets, and nanopowder (from American Elements'nanoscale production facilities). Platinum Oxide is generally immediately available in most volumes. Ultra high purity and high purity compositions improve both optical quality and usefulness as scientific standards. Nanoscale elemental powders and suspensions, as alternative high surface area forms, may be considered. | | Uses | Hydrogenation: Reduction of alkynes to alkenes, Catalyst/ In the presence of H2,PtO2 can be reduced to Pt black which is the active form;
Hydrogenation of nitro compounds to amines;
Hydrogenation of ketones;
The removal of phenyl groups attached to a heteroatom;
Reduction of aromatic ring;
Dehydrogenation: Dehydrogenation of 1,4-diketone to pyridazine;
Oxidation: Oxidation of alcohols to carbonyl compounds;
Others: Resistance, Low resistance range;
Hydrogen absorbing material: Excellent hydrogen absorbing capacity;
Thick film circuit and electronic component: Insoluble in aqueous solutions (water) and extremely stable;
Solid oxide fuel cells and oxygen generation systems: Cathode/certain perovskite structured oxides are electronically conductive. | | References | 1.http://reag.paperplane.io/00002324.htm
2.http://www.sigmaaldrich.com/catalog/product/aldrich/206032?lang=zh®ion=CN
3.https://www.americanelements.com/platinum-dioxide-adams-catalyst-1314-15-4
4.https://en.wikipedia.org/wiki/Adams%27s_catalyst#Uses
5.https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1254558.htm
6.https://www.bipm.org/documents/20126/41773843/Guide_ITS-90_5_SPRT_2021.pdf | | Chemical Properties | Platinum dioxide, also known as Adams catalyst, is a dark brown powder, a hydrated form of platinum (IV) oxide (PtO2), produced by heating chloroplatinic acid (H2PtCl6) with sodium nitrate (NaNO3). Platinum nitrate is produced, and this decomposes to Platinum (IV) oxide with evolution of NO2 and oxygen. It is used in hydrogenations of alkenes to alkanes, nitro compounds to aminos, and ketones to alcohols. The actual catalyst is not the oxide but finely divided platinum black, which forms during the hydrogenation reaction. | | Uses | As catalyst in hydrogenations. The actual catalyst is platinum black which is formed in situ by reduction of the PtO2 by the hydrogen used for the hydrogenation. Especially useful for reduction at room tempereture and hydrogen pressures up to 4 atmospheres. Suitable for the reduction of double and triple bonds, aromatic nuclei, carbonyl groups, nitro groups, and nitriles. | | Uses | Platinum(IV) oxide is used as catalyst for hydrogenation reactions. It is applied in the hydrogenation of alkenes to alkanes, nitro compounds to amines and ketones to alcohols. It is also involved in the hydrogenolysis of cyclopropyl group in to an isopropyl group and in selective oxidation of primary alcohols. | | Application | Platinum (IV) Oxide commonly referred to as Adam’s Catalyst is used frequently in organic synthesis reactions, mainly catalytic hydrogenation. The compound itself shows no cytotoxicity in cells of the pulmonary origin when compared to its PtCl4 counterpart. It has also been used as a catalyst in amperometric biosensors that are based on oxidase enzymes. | | Production Methods | PtO2 is obtained by reduction in chloroplatinic acid with
formaldehyde or by fusing chloroplatinic acid with sodium
nitrate. | | General Description | Platinum(IV) oxide has been used for the reduction of citernyl bromide to form (S)-3,7-dimethyloctylbromide. Prepared by Adams′ nitrate fusion method. |
| | Platinum dioxide Preparation Products And Raw materials |
| Raw materials | Chloroplantinic acid-->Sodium nitrate | | Preparation Products | (2-METHOXY-1-METHYL-ETHYL)-HYDRAZINE-->5-(BENZYLOXY)-1H-PYRROLO[3,2-B]PYRIDINE-2-CARBALDEHYDE-->4-BROMO-2-(TRIFLUOROMETHYL)-5-METHOXYPYRIDINE-->Finasteride-->3-Cyclohexyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyrazine-->2-(2,3-DIHYDRO-1BENZENESULFONYL-PYRROLO[2,3-B]PYRIDIN-3-YL)ETHANAMINE-->Platinum-->2,5-Diaminopyrimidine-->1-Methyl-4-(methylamino)piperidine-->(1,3-DIMETHYL-BUTYL)-HYDRAZINE-->(1,2-DIMETHYL-PROPYL)-HYDRAZINE |
|