1,2,4-Triazole synthesis
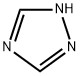
- Product Name:1,2,4-Triazole
- CAS Number:288-88-0
- Molecular formula:C2H3N3
- Molecular Weight:69.07
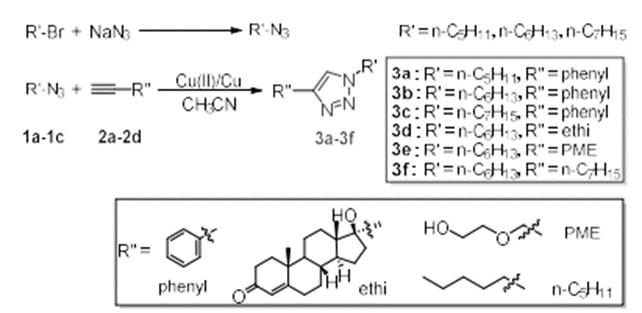 As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.
As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.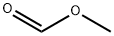
107-31-3
330 suppliers
$16.00/25mL

288-88-0
781 suppliers
$10.00/5g
Yield:288-88-0 94%
Reaction Conditions:
with ammonium chloride;hydrazine hydrate at 120;Sealed tube;Large scale;Reagent/catalyst;Temperature;
Steps:
8.1; 8.2 Embodiment 8
(1) To a 10 L autoclave equipped with a mechanical stirrer, 5.0 kg of methyl formate, 2.0 kg of hydrazine hydrate and 1.3 kg of 30% ammonium chloride were successively added, and the temperature was gradually raised to 120 ° C under a sealed agitation. Hour, the natural cooling and slowly open the vent valve condensation recovery of methanol in the kettle, ammonia and other organic gases. To room temperature to obtain a white emulsion;(2) transferring the white emulsion into a 50L glass reaction kettle, adding 5Kg of 95% ethanol, heating and refluxing to obtain a mixed solution, filtering the mixture to a crystallization kettle through a filter cartridge, cooling the filtrate to a room temperature , Precipitation of white crystals, centrifugal separation, 80 ° C in the hot air oven drying can be obtained 1H-1,2,4-triazole 2.2Kg, hydrazine hydrate yield 94%.
References:
CN105906575,2016,A Location in patent:Paragraph 0074; 0075; 0076;
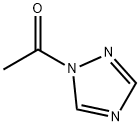
15625-88-4
30 suppliers
$275.00/500mg

288-88-0
781 suppliers
$10.00/5g
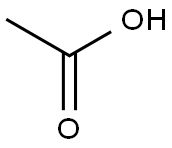
64-19-7
1521 suppliers
$10.00/25ML
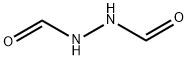
628-36-4
142 suppliers
$12.00/1g

288-88-0
781 suppliers
$10.00/5g