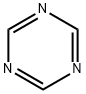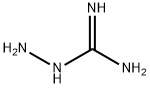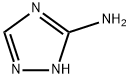
3-Amino-1,2,4-Triazole synthesis
- Product Name:3-Amino-1,2,4-Triazole
- CAS Number:61-82-5
- Molecular formula:C2H4N4
- Molecular Weight:84.08
75232-02-9
20 suppliers
inquiry
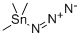
1118-03-2
32 suppliers
$67.09/1g

71831-21-5
118 suppliers
$23.00/100mg

61-82-5
478 suppliers
$10.00/1g
Yield:-
Reaction Conditions:
with ammonia;aminoguanidine in methanol;N,N-dimethyl-formamide;toluene;
Steps:
9 PART II:
In this synthesis, the ester group of intermediate 33 is converted to a nitrile prior to alkylating a heterocycle (Part I) with this substituted benzyl element. Thus, reaction of ester 33 with ammonia in methanol, followed by dehydration of amide 34 produces nitrile 35. Benzylic bromination affords 36, which then may be reacted with the sodium salt of a heterocycle such as 5 in DMF to give an intermediate like 37. Finally, reaction of a nitrile like 37 with trimethylstannyl azide in refluxing toluene gives tetrazoles related to 38 as shown in Scheme II-9. STR57 The preparation of a derivative of Formula I analogous to tetrazole 38 (Scheme II-9) in which X is a methylene group, Y is a single bond and R12 is phenyl, is shown in Scheme II-11. In this synthesis, phenylacetonitrile is deprotonated with lithium bis(trimethylsilyl)amide and then alkylated with the tert-butyldimethylsilylether of p-hydroxymethylbenzyl bromide (preparation of bromide 39 is shown in Scheme II-10) to yield nitrile 40.
References:
US5264439,1993,A

64-18-6
1056 suppliers
$22.12/250ML
2582-30-1
383 suppliers
$18.00/25g

61-82-5
478 suppliers
$10.00/1g
![6-Nitro-[1,2,4]triazolo[1,5-a]pyrimidine](/CAS/20210111/GIF/80772-98-1.gif)
80772-98-1
3 suppliers
inquiry

100-46-9
460 suppliers
$5.00/5G

61-82-5
478 suppliers
$10.00/1g

123767-93-1
1 suppliers
inquiry

107839-38-3
0 suppliers
inquiry

61-82-5
478 suppliers
$10.00/1g