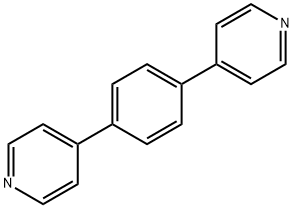
1,4-Di(4-pyridyl)benzene synthesis
- Product Name:1,4-Di(4-pyridyl)benzene
- CAS Number:113682-56-7
- Molecular formula:C16H12N2
- Molecular Weight:232.28
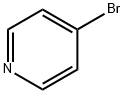
1120-87-2
171 suppliers
inquiry
4612-26-4
280 suppliers
$6.00/1g

113682-56-7
102 suppliers
$20.00/100mg
Yield:113682-56-7 90%
Reaction Conditions:
with C37H45ClN2O3PPd;potassium carbonate in ethanol;water at 80; for 6 h;Inert atmosphere;Schlenk technique;Suzuki Coupling;
Steps:
General procedure for Suzuki coupling reactions
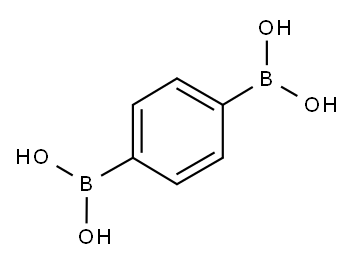
A 10-mL round-bottom flask was charged with the prescribe damount of catalyst, 1,4-benzenediboronic acid (0.5 mmol), N-heteroaryl halides (1.5 mmol), the selected base (1.5 mmol) and solvent (4 mL). The flask was placed in an oil bath and heated at 80 °C for 6 h, then cooled to room temperature and extracted with CH2Cl2. The crude products obtained from evaporation were purified by flash chromatography on silica gel. The products 5b-c, 5f, 5m [21], 5d [22], 5e [23], 5l [24] were known compounds and characterized by the comparison of data with those in the literature. The products 5a, 5g-k, 5n-o were new compounds and characterized by elemental analysis, IR, MS,1H and 13C NMR.
References:
Xiao, Zhi-Qiang;Xu, Chen;Li, Hong-Mei;Han, Xin;Wang, Zhi-Qiang;Fu, Wei-Jun;Hao, Xin-Qi;Song, Mao-Ping [Transition Metal Chemistry,2015,vol. 40,# 5,art. no. 9942,p. 501 - 508]
106-37-6
469 suppliers
$9.00/5g
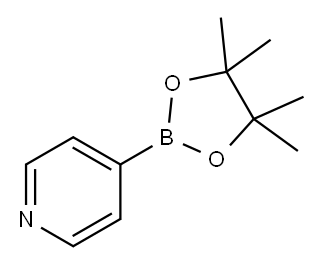
181219-01-2
243 suppliers
$13.00/1g

113682-56-7
102 suppliers
$20.00/100mg
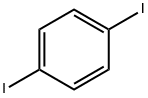
624-38-4
370 suppliers
$6.00/5g
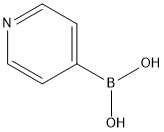
1692-15-5
446 suppliers
$6.00/1g

113682-56-7
102 suppliers
$20.00/100mg

83420-59-1
19 suppliers
inquiry

106-37-6
469 suppliers
$9.00/5g

1692-15-5
446 suppliers
$6.00/1g

113682-56-7
102 suppliers
$20.00/100mg

106-37-6
469 suppliers
$9.00/5g
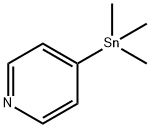
59020-06-3
31 suppliers
$129.00/1g

113682-56-7
102 suppliers
$20.00/100mg