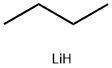
1-Bromobutane synthesis
- Product Name:1-Bromobutane
- CAS Number:109-65-9
- Molecular formula:C4H9Br
- Molecular Weight:137.02
The preparation method of 1-bromobutane is as follows: stirring n-butanol, sodium bromide, water and sulfuric acid evenly, heating under reflux for 3 hours, steaming out the crude bromobutane, washing with water, treating with cold concentrated sulfuric acid, and distilling to obtain the finished product.
Reaction equation: CH3CH2CH2CH2OH+NaBr+H2SO4→CH3CH2CH2CH2Br+Na2SO4+H2O
This halide is easily prepared by reacting butan-1-ol (primary alcohol) with sodium bromide solution and excess of concentrated sulfuric acid. The reaction between sodium bromide and sulphuric acid origins hydrobromic acid.
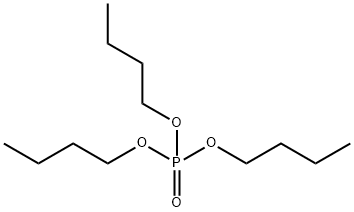
126-73-8
517 suppliers
$6.00/100g

109-65-9
541 suppliers
$10.00/10g
Yield:109-65-9 100%
Reaction Conditions:
with salophen(tBu)AlBr in chloroform-d1 at 20; for 0.5 - 24 h;Product distribution / selectivity;
Steps:
4.C
C.) Salophen(tBu)AlBrIn a vial 30 mg (0.05 mmol) salophen(t)AlBr was dissolved in about 1 mL CDCl3. The solution was transferred to a NMR tube in which trimethyl phosphate (1.81 μL, 0.015 mmol, density 1.197 g/mL) was added with a syringe. The mixture was shaken and monitored by 1H NMR. The % dealkylation was calculated from the peak integrations of methyl bromide produced and unchanged phosphate. TABLE 1 % Dealkylation[a] of trimethyl phosphate (TMP) and tributylphosphate (TBP) with Salen(tBu)AlBr compounds. Salen Salpen Salophen (tBu)AlBr(tBu)AlBr(tBu)AlBr Compound TMP TBP TMP TBP TMP TBP 30 minutes 64 12 35 7 18 100 2 hours 79 100 44 100 30 100 4 hours 89 100 44 100 40 100 6 hours 91 56 46 8 hours 93 58 58 10 hours 95 62 64 12 hours 96 67 69 24 hours 96 100 69 100 85 100 [a]Calculated from the integration of 1H NMR spectra of methyl bromide produced and unchanged trimethyl phosphate at room temperature in CDCl3. In CD3OD after 24 hours salen(tBu)AlBr showed no dealkylation while salpen(tBu)AlBr and salophen(tBu)AlBr had 4% and 3% dealkylation of TMP, respectively.
References:
US7105703,2006,B1 Location in patent:Page/Page column 9

71-36-3
1021 suppliers
$14.00/25mL

109-65-9
541 suppliers
$10.00/10g

5162-44-7
354 suppliers
$10.00/5G

109-65-9
541 suppliers
$10.00/10g
1825-65-6
4 suppliers
inquiry

109-65-9
541 suppliers
$10.00/10g