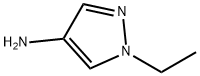
1-ethyl-1H-pyrazol-4-amine synthesis
- Product Name:1-ethyl-1H-pyrazol-4-amine
- CAS Number:876343-24-7
- Molecular formula:C5H9N3
- Molecular Weight:111.15
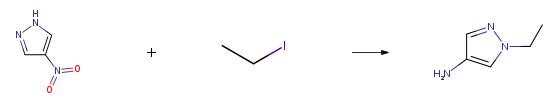
General Procedure for the Synthesis of 4-Amino-l-N-alkylated-pyrazolesA solution of 4-nitropyrazole (300mg, 2.65mmol), potassium carbonate (2eq) and the alkylating reagent (l .leq) in acetonitrile (lOmL) was heated at 60°C for 18h. After cooling to rt the mixture was diluted with EtOAc and washed with water. The organic phase was collected, dried (MgS04) and concentrated in vacuo. The crude residue was dissolved in methanol (lOmL), palladium on carbon (50mg) was added and the reaction was stirred under a balloon of hydrogen for 18h. The resulting mixture was filtered through Celite and the filtrate concentrated in vacuo to give the desired product. Procedure B:Example 11: 2-((6-( 1 -Ethyl- 1 H-pyrazo 1-4-ylamino)- 1 H-pyrazo lo [3 ,4-d]pyrimidin- 1 -yl) methyl)benzonitrileThe following compound was made according to the procedure in Example 1, using 1-ethyl- lH-pyrazo 1-4-amine. 1 -ethyl- lH-pyrazol-4-amine was prepared by Procedure A using ethyl iodide as alkylating agent: 1H NMR (d6-DMSO) δ 9.94 (s, 1H), 8.94 (s, 1H), 8.10 (s, 2H), 7.91 (dd, 1H), 7.66 (td, 1H), 7.49-7.53 (m, 2H), 7.35-7.37 (m, 1H), 5.76 (s, 2H), 4.11 (q, 2H), 1.35 (t, 3H); LC-MS method B, (ES+) 345.1, RT = 8.58min.

58793-45-6
33 suppliers
inquiry

876343-24-7
78 suppliers
$47.00/500mg
Yield:876343-24-7 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol; under 2585.81 Torr; for 3 h;Inert atmosphere;
Steps:
111b
Example 111b l-Ethyl-lH-pyrazol-4-amine 111b CGI PHARM6 0WOA 250-mL Parr reactor bottle was purged with nitrogen and charged with 10% palladium on carbon (50% wet, 468 mg dry weight) and a solution of 111a (1.90 g, 13.5 mmol) in ethanol (100 mL). The bottle was attached to a Parr hydrogenator, evacuated, charged with hydrogen gas to a pressure of 50 psi and shaken for 3 h. After this time, the hydrogen was evacuated, and nitrogen was charged into the bottle. Celite 521 (1.00 g) was added, and the mixture was filtered through a pad of Celite 521. The filter cake was washed with ethanol (2 x 25 mL), and the combined filtrates were concentrated to dryness under reduced pressure to afford a quantitative yield of 111b (1.50 g) as a purple oil: ]H NMR (300 MHz, CDC13) δ 7.15 (s, 1H), 7.02 (s, 1H), 4.05 (q, 2H, / = 7.2 Hz), 2.88 (br s, 2H), 1.43 (t, 3H, / = 7.2 Hz); MS (ESI+) m/z 112.1 (M+H).
References:
WO2012/31004,2012,A1 Location in patent:Page/Page column 84-85
2075-46-9
309 suppliers
$6.00/5g

75-03-6
357 suppliers
$11.00/5g

876343-24-7
78 suppliers
$47.00/500mg