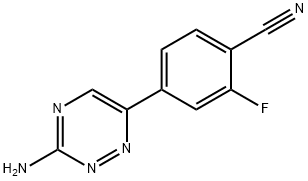
4-(3-Amino-1,2,4-triazin-6-yl)-2-fluorobenzonitrile synthesis
- Product Name:4-(3-Amino-1,2,4-triazin-6-yl)-2-fluorobenzonitrile
- CAS Number:1197377-47-1
- Molecular formula:C10H6FN5
- Molecular Weight:215.19
870238-67-8
100 suppliers
$11.00/250mg
69249-22-5
132 suppliers
$12.00/250mg

1197377-47-1
68 suppliers
inquiry
Yield: 82.3%
Reaction Conditions:
with potassium carbonate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane;water at 20 - 86;Inert atmosphere;
Steps:
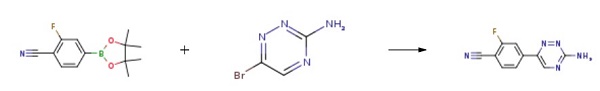
25 4-(3-Amino-1,2,4-triazin-6-yl)-2-fluorobenzonitrile (20)
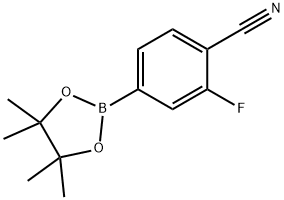
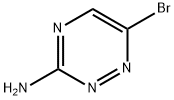
Example 25 4-(3-Amino-1,2,4-triazin-6-yl)-2-fluorobenzonitrile (20) A mixture of 6-bromo-1,2,4-triazin-3-amine (17, 100.0 g, 571.47 mmol) and 2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile (19, 145.43 g, 588.61 mmol, 1.03 equiv) in 1,4-dioxane (1200 mL) was stirred at room temperature for 10 min before potassium carbonate (K2CO3, 355.4 g, 2572 mmol) in water (600 mL) was added to give a deep red solution. The mixture was degassed by bubbling with nitrogen for 10 min before 1,1'-bis(diphenyl phosphino)ferrocene dichloropalladium(II) complex with dichloromethane (1:1) (Pd(dppf)2Cl2, 14.14 g, 17.14 mmol, 0.03 equiv) was added at room temperature. The resulting reaction mixture was degassed by bubbling with nitrogen for 10 min and then heated at 86° C. under nitrogen. After 2 h, HPLC showed that the reaction was deemed complete, and the reaction mixture was cooled to room temperature and then to 0-5° C. with an ice-water bath. 1,4-Dioxane (400 mL) was added to the cooled reaction mixture before a solution of 3.3 M aqueous hydrochloric acid solution (HCl, 1900 mL) was added dropwise with stirring to adjust pH to 0.40-0.93. The mixture was stirred at room temperature for 30 min and filtered. The solid collected was stirred with 1,4-dioxane (260 mL) and then added 1N HCl (400 mL). The mixture was stirred at room temperature for 10 min and filtered. The filtrate was combined with the filtrate obtained earlier and washed with ethyl acetate (EtOAc, 2*2 L). The combined ethyl acetate extracts was extracted with 1 N aqueous hydrochloric acid solution (HCl, 3*200 mL). The combined aqueous solution was then treated with activated charcoal (20 g) and stirred at room temperature for 30 min. The mixture was filtered through a celite bed and the filtrate was cooled to 0-5° C. with an ice-water bath. A solution of 50% of sodium hydroxide in water (NaOH, 240 mL, 4500 mmol) was added dropwise at 5-12° C. to adjust pH to 10.6-11.6. The mixture was stirred at 0-5° C. for 30 min and then filtered. The solids collected were washed with aqueous ammonium hydroxide (1 to 3 of 28% concentrated NH4OH to water, 1900 mL) and dried under vacuum at 40-45° C. to constant weight to afford 4-(3-amino-1,2,4-triazin-6-yl)-2-fluorobenzonitrile (20, 101.2 g, 122.9 g theoretical, 82.3% yield) as a off-white powder.
References:
Location in patent:Page/Page column 25; 26