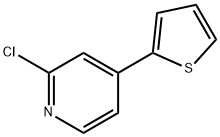
2-chloro-4-(thiophen-2-yl)pyridine synthesis
- Product Name:2-chloro-4-(thiophen-2-yl)pyridine
- CAS Number:1289555-51-6
- Molecular formula:C9H6ClNS
- Molecular Weight:195.67
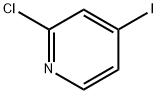
153034-86-7
360 suppliers
$10.00/1g
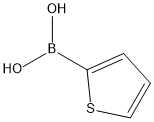
6165-68-0
382 suppliers
$14.00/5g

1289555-51-6
13 suppliers
inquiry
Yield:1289555-51-6 88%
Reaction Conditions:
with sodium carbonate;trans-bis(triphenylphosphine)palladium dichloride in tetrahydrofuran;water at 70;
Steps:
113.1
Step 1 A round-bottomed flask was charged with 2-chloro-4-iodopyridine (600 mg, 2.5 mmol), thiophen-2-ylboronic acid (385 mg, 3.0 mmol), trans-dichlorobis(triphenylphosphine)palladium (II) (176 mg, 0.251 mmol), THF (9 mL) and 2M aqueous sodium carbonate (3.0 mL, 6.0 mmol). The reaction mixture was stirred at 70° C. overnight. The reaction mixture was cooled to room temperature then diluted with 20 mL water and extracted with 100 mL EtOAc (2*). The combined organic layers were washed with 20 mL water and 20 mL brine then dried over sodium sulfate, filtered and concentrated. The residue was absorbed on ~2 g SiO2 and chromatographed over 24 g SiO2 with EtOAc/hexanes (gradient: 0-10% EtOAc). All fractions containing product were combined and concentrated to give 430 mg (88%) of 2-chloro-4-thiophen-2-yl-pyridine as a light yellow solid.
References:
US2011/230462,2011,A1 Location in patent:Page/Page column 98
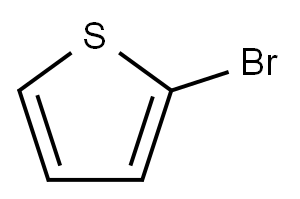
1003-09-4
467 suppliers
$10.00/25g
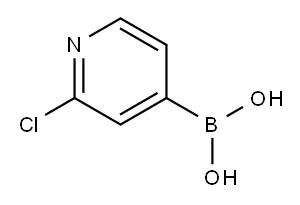
458532-96-2
265 suppliers
$6.00/250mg

1289555-51-6
13 suppliers
inquiry

153034-86-7
360 suppliers
$10.00/1g
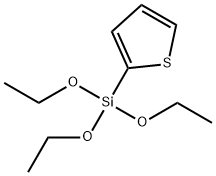
17984-89-3
21 suppliers
$61.20/1g

1289555-51-6
13 suppliers
inquiry
![2,2'-DICHLORO-[4,4']-BIPYRIDINE](/CAS/GIF/53344-74-4.gif)
53344-74-4
42 suppliers
$86.90/100mg
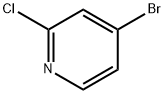
73583-37-6
319 suppliers
$9.00/1g

6165-68-0
382 suppliers
$14.00/5g

1289555-51-6
13 suppliers
inquiry