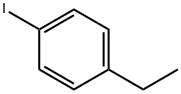
2-ETHYLIODOBENZENE synthesis
- Product Name:2-ETHYLIODOBENZENE
- CAS Number:18282-40-1
- Molecular formula:C8H9I
- Molecular Weight:232.06
578-54-1
254 suppliers
$10.00/5g

18282-40-1
107 suppliers
$8.00/5g
Yield: 85%
Reaction Conditions:
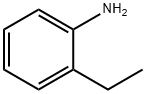
Stage #1:ortho-ethylaniline with sulfuric acid;sodium nitrite in water at -20; for 1 h;
Stage #2: with potassium iodide in water at 20; for 18 h;
Steps:
14 2-Ethyl-iodobenzene
With mechanical stirring, a suspension of 75 2-ethylaniline (36.4 g, 0.30 mol) in 25 wt % 76 sulfuric acid (240 mL), was cooled in 1, 2-xylene-dry ice bath to -20° C. 77 Sodium nitrite (21 g, 0.30 mol) in 10 water (40 mL) was added, and after 1 hour at this temperature, the gelly mass was transferred to a solution of 14 potassium iodide (150 g, 0.90 mol) in water (150 mL), and the mixture was left stirring at room temperature for 18 hours. The reaction 78 mass was extracted into hexanes and the extract after drying was passed through a column of silica gel to get the product as a colorless oil after evaporation of the solvent, 59.14 g (85%). Mass spec: m/z 232 (M+). Anal. Calcd. for C8H9I: C, 41.41; H, 3.91 and I, 54.32%. Found: C, 41.50; H, 3.90 and I, 54.28%. 1H NMR (CDCl3) δ ppm: 1.20 (t, 3H, 7.52 Hz), 2.73 (q, 2H, 7.52 Hz), 6.86 (td, 1H, 7.50 and 1.76 Hz), 7.25 (m, 2H), 7.80 (dd, 1H, 0.88 and 7.88 Hz).
References:
The United States of America, as represented by the Secretary of the Air Force;Tan, Loon-Seng;Kannan, Ramamurthi US10113065, 2018, B1 Location in patent:Page/Page column 18
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

18282-40-1
107 suppliers
$8.00/5g

128254-32-0
0 suppliers
inquiry

18282-40-1
107 suppliers
$8.00/5g

59473-45-9
79 suppliers
$35.00/1g

18282-40-1
107 suppliers
$8.00/5g