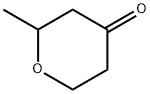
2-methyloxan-4-one synthesis
- Product Name:2-methyloxan-4-one
- CAS Number:1193-20-0
- Molecular formula:C6H10O2
- Molecular Weight:114.14
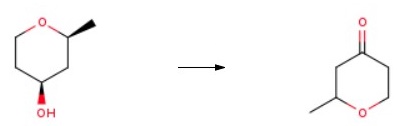
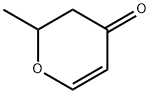
19185-89-8
3 suppliers
inquiry

1193-20-0
85 suppliers
$45.00/50mg
Yield:1193-20-0 57%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 20; for 72 h;
Steps:
4.b
1.35 g of 2 -methyl-2, 3-dihydropyran-4-one in 15 ml of ethyl acetate, in the presence of 270 mg of palladium-on-charcoal at 10%, are stirred under a hydrogen atmosphere at ambient temperature for 3 days. The reaction medium is filtered through filter paper and the filtrate is evaporated. The residue is chromatographed on silica gel, elution being carried out with 60/40 pentane/diethyl ether.787 mg of 2-methyltetrahydropyran-4-one are obtained in the form of a yellow oil. Yield = 57%.
References:
WO2010/63773,2010,A1 Location in patent:Page/Page column 20-21

89791-47-9
53 suppliers
$22.00/250mg

1193-20-0
85 suppliers
$45.00/50mg

821-08-9
5 suppliers
inquiry

1193-20-0
85 suppliers
$45.00/50mg