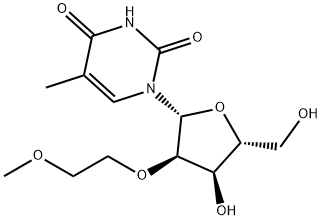
2'-O-(2-Methoxyethyl)-5-methyluridine synthesis
- Product Name:2'-O-(2-Methoxyethyl)-5-methyluridine
- CAS Number:163759-49-7
- Molecular formula:C13H20N2O7
- Molecular Weight:316.31
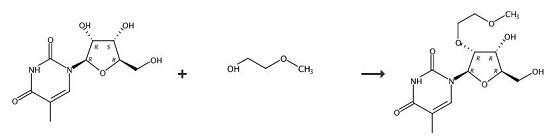
2,2'-Anhydro-5-methyluridine (195 g, 0.81 M), tris(2-methoxyethyl)borate (231 g, 0.98 M) and 2-methoxyethanol (1.2 L) were added to a 2 L stainless steel pressure vessel and placed in a pre-heated oil bath at 160°C. After heating for 48 hours at 155-160°C, the vessel was opened and the solution evaporated to dryness and triturated with methanol (200 mL). The residue was suspended in hot acetone (1 L). The insoluble salts were filtered, washed with acetone (150 mL) and the filtrate evaporated. The residue (280 g) was dissolved in CH3CN (600 mL) and evaporated. A silica gel column (3 kg) was packed in CH2Cl2/ acetone/methanol (20:5:3) containing 0.5% Et3NH. The residue was dissolved in CH2Cl2 (250 mL) and adsorbed onto silica (150 g) prior to loading onto the column. The product was eluted with the packing solvent to give product. Yield 160 g, 63%.
22423-26-3
198 suppliers
$9.00/1g

109-86-4
518 suppliers
$11.00/25g

163759-49-7
104 suppliers
inquiry
Yield:163759-49-7 91%
Reaction Conditions:
with Orthoboric acid;Sodium hydrogenocarbonate in toluene at 150; for 14 h;Solvent;
Steps:
1-4; 11 Example 1 Preparation of 2'-O-substituted-5-methyluridine (compound a)
General procedure: In a 250mL round-bottom flask, add the compound of formula II 2,2'-anhydro-5-methyluridine (12g, 0.050mol, 1.0equiv.), boric acid (0.62g, 0.010mol, 0.20equiv.), sodium bicarbonate (0.84 g, 0.010 mol, 0.20 equiv.), 50 mL of compound 1 of formula III, 20 mL of toluene, equipped with a thorn-shaped distillation column, heated at 150° C. for 14 h, and monitored by TLC until the reaction was complete.Placed in a rotary evaporator and concentrated to dryness under reduced pressure to obtain compound a.The prepared compound a was purified by column chromatography, and the column chromatography eluent was dichloromethane:methanol=20:1.After separation and purification by column chromatography, compound a (14.42 g, yield 91%, purity>95%) was obtained.
References:
CN114621303,2022,A Location in patent:Paragraph 0047-0062; 0111-0112
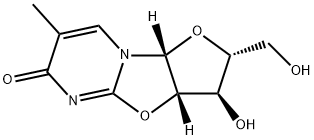
![6H-Furo[2',3':4,5]oxazolo[3,2-a]pyrimidin-6-one, 2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxymethyl)-7-methyl-, (2R,3R,3aR,9aR)-](/CAS/20210305/GIF/947322-40-9.gif)
947322-40-9
0 suppliers
inquiry

14983-42-7
30 suppliers
inquiry

109-86-4
518 suppliers
$11.00/25g

163759-49-7
104 suppliers
inquiry
![Uridine, 3',5'-bis-O-[(2,4-dichlorophenyl)methyl]-2'-O-(2-methoxyethyl)-5-methyl- (9CI)](/CAS/20210305/GIF/168427-88-1.gif)
168427-88-1
0 suppliers
inquiry

163759-49-7
104 suppliers
inquiry
![6H-Furo[2',3':4,5]oxazolo[3,2-a]pyrimidin-6-one, 2,3,3a,4a,5,9a-hexahydro-3-hydroxy-2-(hydroxymethyl)-7-methyl-, (2R,3R,3aS,9aR)-](/CAS/20210111/GIF/215713-37-4.gif)