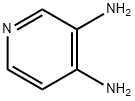
3,4-Diaminopyridine synthesis
- Product Name:3,4-Diaminopyridine
- CAS Number:54-96-6
- Molecular formula:C5H7N3
- Molecular Weight:109.13

Reference: Bakke, J. M.; Riha, J. Synthesis of 3,4-diaminopyridine and imidazo[4,5-c]pyridines by nitration of 4-(acylamino)pyridines. Journal of Heterocyclic Chemistry. Volume 36. Issue 5. Pages 1143-1145. Journal. (1999)
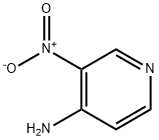
1681-37-4
332 suppliers
$5.00/1g

54-96-6
347 suppliers
$5.00/100mg
Yield:54-96-6 97%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in tetrahydrofuran at 10; under 760.051 Torr; for 24 h;
Steps:
1 Step 1 : synthesis of pyridine-3,4-diamine 8
The commercially available 3-nitropyridin-4-amine (50 g, 395 mmol) in the mixture of methanol (500 ml) and THF (500 ml) was hydrogenated with 10 % Pd/C (5 g) as a catalyst at 10°C (1 atm) for 24 h. After uptake of (3 eq), the catalyst was filtered off and the filtrate was evaporated. 38 g of the title intermediate 8 was obtained, (Yield 97 %).
References:
JANSSEN R&D IRELAND;TAHRI, Abdellah;JONCKERS, Tim Hugo Maria;RABOISSON, Pierre Jean-Marie Bernard;DEMIN, Samuël Dominique WO2014/114776, 2014, A1 Location in patent:Page/Page column 25
33544-42-2
68 suppliers
$45.00/50mg

54-96-6
347 suppliers
$5.00/100mg

100306-70-5
53 suppliers
inquiry

54-96-6
347 suppliers
$5.00/100mg
626-64-2
506 suppliers
$6.00/5g

54-96-6
347 suppliers
$5.00/100mg
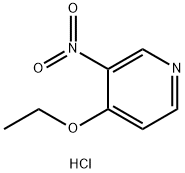
94602-04-7
67 suppliers
$37.79/5g

54-96-6
347 suppliers
$5.00/100mg


