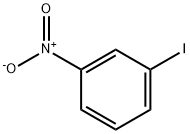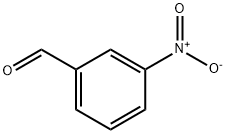
3-Nitrobenzaldehyde synthesis
- Product Name:3-Nitrobenzaldehyde
- CAS Number:99-61-6
- Molecular formula:C7H5NO3
- Molecular Weight:151.12
The reaction mixture is poured in a 1 L beaker on 500 g crunched ice, the yellow precipitation is sucked off at 16 hPa over a Buechner funnel and washed with 200 mL of cold water. Crude yield (humid): 14.4 g
The humid crude product is dissolved in 125 mL tert-butyl methyl ether and then shaken out with 125 mL of a 5% NaHCO3 solution. The organic phase is dried over sodium sulfate, filtered and the solvent evaporated at a rotary evaporator. The residue is recrystallized from toluene / petroleumether (60-80 °C) by dissolving it in toluene whilst heating and then adding the double amount of petroleum ether in portions under ice cooling. The crystalllized light yellow 3-nitrobenzaldehyde is sucked off over a Buechner funnel. The product is dried over silica gel in an evacuated desiccator.
Yield: 8.0 g (53 mmol, 53%); mp 56 °C
99-08-1
204 suppliers
$5.00/5G

99-61-6
630 suppliers
$5.00/10g
Yield:99-61-6 98%
Reaction Conditions:
with laccase of Pleurotus ostreatus MTCC-1801;2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt in 1,4-dioxane at 20; pH=4.5;Enzymatic reaction;
Steps:
Bioconversions in the Presence of ABTS
General procedure: The biotransformation of toluene to benzaldehyde [32-34] was done in 15mLof 100mM sodium acetate buffer pH 4.5 containing 20mM toluene in dioxane,0.1mM ABTS, and 500 mL of twice-diluted crude laccase (activity of concentratedlaccase was 1.91 IU=mL) kept in a 100-mL conical flask, which was stirred vigorouslyfor 60 min (completion of the reaction was confirmed by the UV=vis spectrophotometer(Hitachi, Japan, model U-2900). The reaction solution was extractedthree times with 40mL of ethyl acetate, and 20 mL of the n-ethyl acetate extractwas injected in Waters HPLC model 600E using spherisorb C18 5 UV, 4.5250mmmm column. The mobile phase was methanol at the flow rate of 0.5mL=min. Thedetection was made using Waters UV detector model 2487 at k254 nm.
References:
Chaurasia, Pankaj Kumar;Yadava, Sudha;Bharati, Shashi Lata;Singh, Sunil Kumar [Synthetic Communications,2014,vol. 44,# 17,p. 2535 - 2544]

29949-19-7
2 suppliers
inquiry

99-61-6
630 suppliers
$5.00/10g
3958-57-4
237 suppliers
$14.00/10g

99-61-6
630 suppliers
$5.00/10g
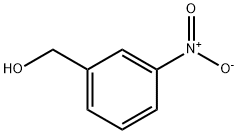
619-25-0
213 suppliers
$8.00/5g

99-61-6
630 suppliers
$5.00/10g