
4-amino-N-(2-fluorophenyl)benzenesulfonamide synthesis
- Product Name:4-amino-N-(2-fluorophenyl)benzenesulfonamide
- CAS Number:1494-83-3
- Molecular formula:C12H11FN2O2S
- Molecular Weight:266.29
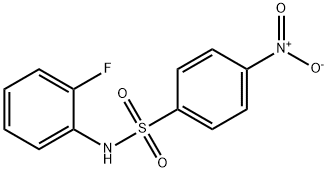
1766-57-0
2 suppliers
inquiry

1494-83-3
7 suppliers
$57.50/250mg
Yield:1494-83-3 70%
Reaction Conditions:
with iron;ammonium chloride in methanol;water at 65;
Steps:
10 Example 10
Example 10
N-(2,4-dimethoxyphenyl)-4-aminobenzenesulfonamide
N-(2,4-dimethoxyphenyl)-4-nitrobenzenesulfonamide (11.15 mmol, 3.30 g) was dissolved in methanol (125 mL).
Ammonium chloride (113 mmol, 6 g), dissolved in distillated water (67 mL), and iron (65.33 mmol, 3.65 g) were then added to the reaction mixture.
After stirring overnight at 65 °C, the reaction mixture was filtered through a pad of celite on sintered funnel.
After successive washings with acetone, DCM and ethyl acetate, the biphasic mixture was separated.
The aqueous layer was extracted twice with DCM.
The combined organic layers were dried over MgSO4 and the solvents were concentrated under vacuum.
The crude was purified by chromatography over silica gel affording N-(2,4-dimethoxyphenyl)-4-nitrobenzenesulfonamide (7.89 mmol, 2.10 g) with 70% yield. (Rf: 0.44 (DCM 100%); mp = 190°C). RMN 1H (300 MHz, DMSO-d6): 8.41 (s, 1H, NH), 7.34 (d, 2H, H2-H3), 7.21-7.25 (m, 1H, H6), 7.09-7.12 (m, 3H, H5-H7-H8), 6.54 (d, 2H, H1-H4), 5.96 (s, 2H, NH2). RMN 13C (75 MHz, DMSO-d6): [154.0 - 156.4] (C-F), 152.9 (CIV), 128.6 (C2-C3), [126.3 - 126.4] (Car), 125.6 (C6), [125.2 - 125.3] (CIV), 124.7 (CIV), [124.3 - 124.4] (CIV), [115.7 - 115.9] (C8), 112.5 (C1-C4). HRMS: Calculated for [M+Na]+ 289.0423; Measured: 289.0435 IR (cm-1): 3402 (v NHar), 3337 (vs NH2ar), 1644, 1592, 1495, 1318 (vas SO2), 1148, 1090.
References:
EP3412652,2018,A1 Location in patent:Paragraph 0081-0082
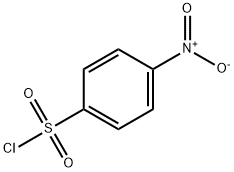
98-74-8
155 suppliers
$10.00/5g

1494-83-3
7 suppliers
$57.50/250mg
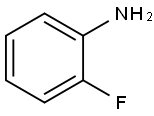
348-54-9
415 suppliers
$10.00/1g

1494-83-3
7 suppliers
$57.50/250mg