4-BROMO-3-FLUORO-4'-METHOXYBENZOPHENONE synthesis
- Product Name:4-BROMO-3-FLUORO-4'-METHOXYBENZOPHENONE
- CAS Number:161581-93-7
- Molecular formula:C14H10BrFO2
- Molecular Weight:309.13

192436-83-2
76 suppliers
$19.00/1g

161581-93-7
9 suppliers
$294.00/1g
Yield:161581-93-7 51%
Reaction Conditions:
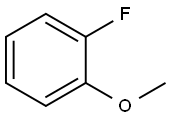
Stage #1: 3-fluoro-4-methoxyphenyl bromidewith n-butyllithium in tetrahydrofuran;hexanes at -78; for 1 h;
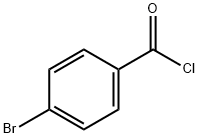
Stage #2: 4-bromo-N-methoxy-N-methylbenzamide in tetrahydrofuran;hexanes at -20; for 1 h;
Steps:
28.2
To a stirred solution of 2-fluoro-4-bromoanisole (3.03 g, 14.8 mmol) in anhydrous THF (100 mL) was added n-butyllithium (1.6 M in hexanes, 10.2 mL, 16.3 mol) dropwise at-78 °C. The reaction mixture was stirred for 1 h and 80 (4.02 g, 16.3 mmol) dissolved in THF (20 mL) was added dropwise. The reaction mixture was warmed to-20 °C over 1 h, water (100 mL) was added, and the volatiles were removed under reduced pressure. The mixture was extracted with ether (3 x 100 mL) and the combined extracts were washed with water (100 mL) and brine (100 mL). The mixture was dried over MgS04 and concentrated. The residue was chromatographed on silica gel (20: 1 to 5: 1 hexanes: EtOAc) to afford 2.35 g (51%) of 81 as a white crystalline SOLID. H NMR (400 MHz, CDCI3) : 8 3.97 (s, 3H), 7.01 (t, J = 8.4 Hz, 1 H), 7.55-7. 62 (m, 6H).
References:
WO2005/12220,2005,A2 Location in patent:Page/Page column 80-81