
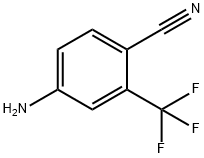
4-isothiocyanato-2-(trifluoroMethyl)benzonitrile synthesis
- Product Name: 4-isothiocyanato-2-(trifluoroMethyl)benzonitrile
- CAS Number:143782-23-4
- Molecular formula:C9H3F3N2S
- Molecular Weight:228.19

4-Amino-2-trifluoromethylbenzonitrile, (2.23 g, 12 mmol) was added portionwise over15 minutes into the well-stirred heterogeneous mixture of thiophosgene (1 ml, 13 mmol) in water (22 ml) at room temperature. Stirring was continued for an additional 1 h. The reaction medium was extracted with chloroform (3 x 15 ml). The combined organic phase was dried over MgSO4 and evaporated to dryness under reduced pressure to yield desired product, 4-isothiocyanato-2-trifluoromethylbenzonitrile, (Ia), as brownish solid and was used as such for the next step (2.72 g, 11.9 mmol, 99%).
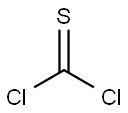
463-71-8
139 suppliers
$32.00/10g
654-70-6
467 suppliers
$5.00/1g

143782-23-4
397 suppliers
$60.00/1g
Yield:143782-23-4 99%
Reaction Conditions:
in lithium hydroxide monohydrate at 20; for 1.25 h;
Steps:
1; 56
4-Amino-2-trifluoromethylbenzonitrile, (2.23 g, 12 mmol) was added portionwise over15 minutes into the well-stirred heterogeneous mixture of thiophosgene (1 ml, 13 mmol) in water (22 ml) at room temperature. Stirring was continued for an additional 1 h. The reaction medium was extracted with chloroform (3 x 15 ml). The combined organic phase was dried over MgSO4 and evaporated to dryness under reduced pressure to yield desired product, 4-isothiocyanato-2-trifluoromethylbenzonitrile, (Ia), as brownish solid and was used as such for the next step (2.72 g, 11.9 mmol, 99%).
References:
WO2006/124118,2006,A1 Location in patent:Page/Page column 21; 79

654-70-6
467 suppliers
$5.00/1g

143782-23-4
397 suppliers
$60.00/1g

654-70-6
467 suppliers
$5.00/1g
6160-65-2
405 suppliers
$6.00/1g

143782-23-4
397 suppliers
$60.00/1g