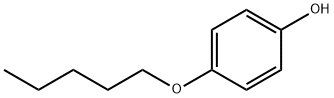
4-Pentyloxyphenol synthesis
- Product Name:4-Pentyloxyphenol
- CAS Number:18979-53-8
- Molecular formula:C11H16O2
- Molecular Weight:180.24
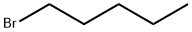
110-53-2
397 suppliers
$12.50/5g
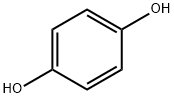
123-31-9
824 suppliers
$13.00/25g

18979-53-8
97 suppliers
$41.00/2 g
Yield: 36%
Reaction Conditions:
with potassium hydroxide in dimethyl sulfoxide at 40; for 3 h;Inert atmosphere;
Steps:
4-Pentyloxyphenol (3e)
4-Pentyloxyphenol (3e) was obtained by a slightly modified procedure [20]. To a solution of 11.01 g (100 mmol) of 1,4-hydroquinone in 130 mL of DMSO under an argon atmosphere was added at vigorous stirring 6.17 g (110 mmol) of powdered KOH. The reaction mixture turned yellow, and thereto was added dropwise a solution of 15.1 g (100 mmol) of 1-bromopentane in 30 mL of DMSO within 0.5 h. The reaction mixture was stirred for 3 h at 40°C (TLC monitoring; Rf of the reaction product ~0.58, eluent hexane-EtOAc, 6 : 4). Then the reaction mixture was poured in 400 mL of cold water, acidified to pH 1 with dilute hydrochloric acid. The precipitate was filtered off and washed with cold water, then it was subjected to column chromatography on silica gel (eluent hexane-CH2Cl2, 9 : 1-4 : 6). The obtained oily substance was crystallized from hexane at cooling. Yield 36%, white needle crystals, mp 46.5-47°C(hexane). 1H NMR spectrum, δ, ppm: 0.93 t (3H, CH3,J 7.2 Hz), 1.33-1.47 m [4H, CH2(CH2)2CH3], 1.76 quintet (2H, CH2CH2CH2, J 7.0 Hz), 3.90 t (2H, CH2, J 6.6 Hz), 4.20 br.s (1H, OH), 6.73-6.81 m (4Harom). Mass spectrum, m/z (Irel, %): 180 (11) [M]+, 144 (4), 110 (100), 81 (8), 65 (5), 53 (5), 43 (18).
References:
Fayzullin;Antonovich;Zakharychev;Bredikhina;Kurenkov;Bredikhin [Russian Journal of Organic Chemistry,2015,vol. 51,# 2,p. 202 - 209][Zh. Org. Khim.,2015,vol. 51,# 2,p. 214 - 221]
543-59-9
185 suppliers
$20.00/25ml

123-31-9
824 suppliers
$13.00/25g

18979-53-8
97 suppliers
$41.00/2 g

71-41-0
425 suppliers
$9.33/100 mL

123-31-9
824 suppliers
$13.00/25g

18979-53-8
97 suppliers
$41.00/2 g